KFEB
| Elnök: | Dr. Bitter István |
| Alelnök: | Dr. Fürst Zsuzsanna |
| Titkárok: | Dr. Arányi Péter Dr. Reusz György |
| Titkárság: | Jegesi Bernadett Kovács Csaba Antalné Edit Baski Barnabás |
| Székhely címe: | 1054 Budapest, Báthory u. 10. |
| Levelezési cím: | Belügyminisztérium, 1903 Budapest, Pf.: 314 |
| Telefon: | (+36 1) 795-4873 (+36 1) 795-1195 (+36 1) 795-6549 |
| E-mail: | kfebtitkarsag@bm.gov.hu |
| SUSAR beküldéséhez: | safetyreport@bm.gov.hu |
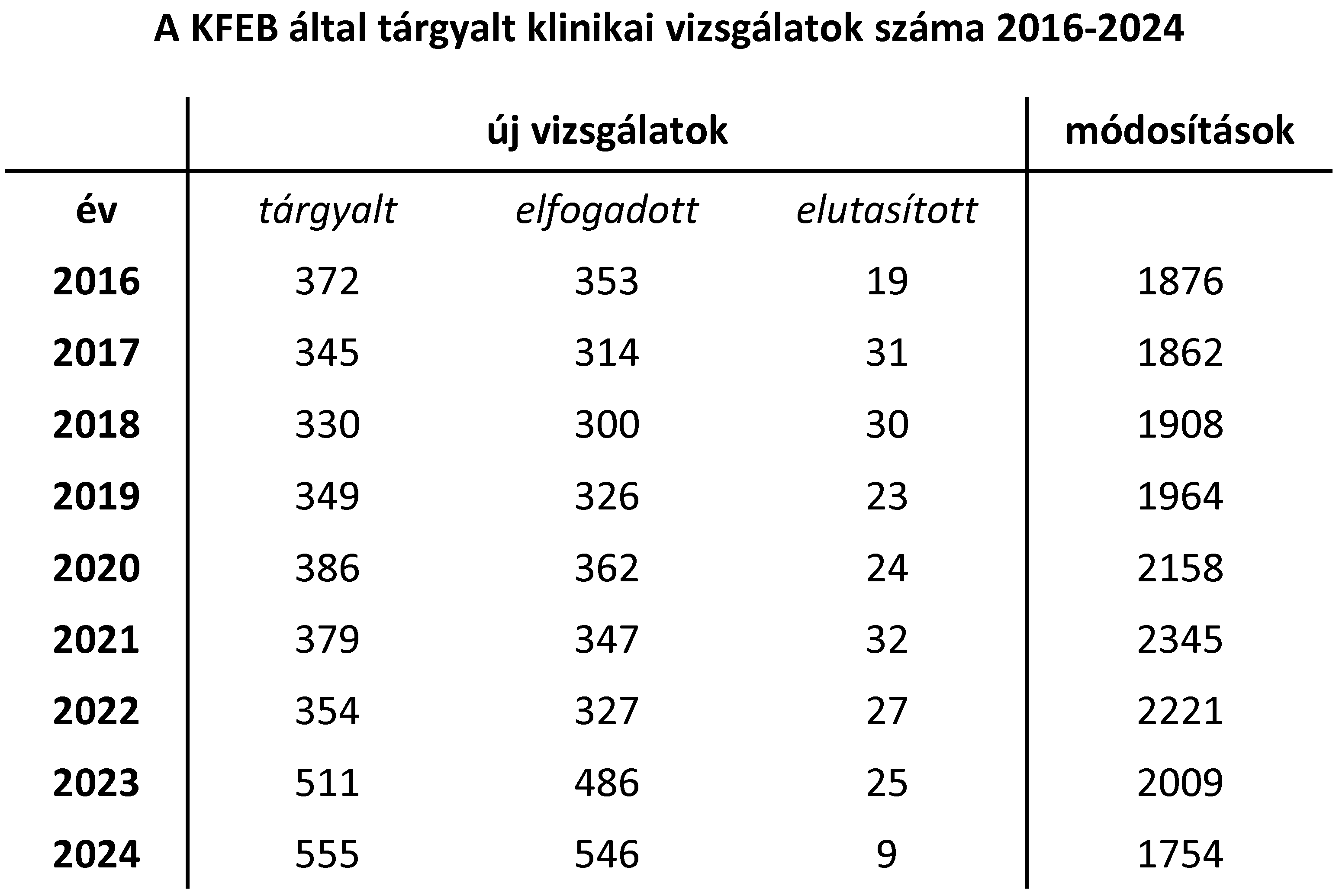
| Tárgyalt kérelmek 2024-ben: |
Új vizsgálat: 555 darab Módosítás: 1754 darab |
A Bizottság tagjai
| Elnök: | Dr. Bitter István pszichiáter, neurológus, klinikai farmakológus |
| Alelnök: | Dr. Fürst Zsuzsanna farmakológus, klinikai farmakológus |
| Titkárok: | Dr. Arányi Péter biokémikus Dr. Reusz György csecsemő- és gyermekgyógyász, nefrológus, hipertonológus |
| Tagok: | Dr. Blaskó György belgyógyász, klinikai farmakológus, gasztroenterológus Dr. Borsodi Anna biokémikus Dr. Czobor Pál biológus, klinikai biostatisztikus Dr. Dobó István sebész Dr. Egri László kézsebész, traumatológus, teológus Dr. Farsang Csaba belgyógyász, nefrológus, hipertonológus, klinikai farmakológus Dr. Gyurkovits Kálmán csecsemő- és gyermekgyógyász, gyermektüdőgyógyász, klinikai farmakológus, gasztroenterológus Dr. Herold Róbert pszichiáter Dr. Hunyady Béla belgyógyász, gasztroenterológus, klinikai farmakológus Dr. Hunyadi János bőrgyógyász, allergológus, klinikai immunológus Dr. Kiss Róbert Gábor kardiológus, belgyógyász Dr. Ludwig Endre infektológus, belgyógyász, klinikai farmakológus Dr. Mangel László Csaba pszichiáter, klinikai onkológus, radioterapeuta Dr. Maráz Anikó sugárterapeuta, klinikai onkológus, klinikai farmakológus Dr. Nagy Ágnes hematológus, belgyógyász Dr. Nagy Zsuzsanna belgyógyász, klinikai onkológus Dr. Orodán Mária jogász Dr. Orosz Gábor Béla teológus, lelkész Dr. Póka Róbert nőgyógyász, klinikai onkológus Dr. Réthelyi János pszichiáter, klinikai genetikus Dr. Rosta András onkológus, hematológus, belgyógyász Dr. Sipos Ildikó neurológus Dr. Szaleczky Erika onkológus, hematológus, belgyógyász Szántai Anita speciális pedagógia szaktanár Dr. Szűcs Nikolette endokrinológus, belgyógyász Dr. Tarnai Julianna reumatológus, klinikai farmakológus |
| Állandó meghívottak: | Dr. Hajdú Ágnes Zita NNGYK |
A Külső Szakértői Bizottság tagjai
Dr. Bereczki Dániel neurológus, pszichiáter
Dr. Borvendég János belgyógyász, klinikai farmakológus
Dr. Csiba László neurológus, pszichiáter
Dr. Demetrovics Zsolt alkalmazott egészségpszichológiai szakpszichológus
Dr. Fülesdi Béla neurológus, aneszteziológus
Dr. Gera István fogorvos, parodontológus
Dr. Járay Zoltán belgyógyász, kardiológus, klinikai farmakológus
Dr. Julesz János belgyógyász, endokrinológus
Dr. Kemény Lajos bőrgyógyász, allergológus
Dr. Kiss Emese Virág reumatológus, belgyógyász, allergológus-klinikai immunológus
Dr. Komoly Sámuel neurológus
Dr. Mészner Zsófia csecsemő- és gyermekgyógyász, infektológus
Dr. Molnár Márk neurológus, pszichofiziológus
Dr. Nagy Zoltán Zsolt szemész
Dr. Németh Péter immunológus
Dr. Péley Bernadette pszichológus
Dr. Prinz Gyula belgyógyász, infektológus
Dr. Sallay Péter gyermekgyógyász, nefrológus
Dr. Sebeszta Miklós belgyógyász, kardiológus
Dr. Somogyi Anikó belgyógyász, klinikai farmakológus, endokrinológus
Dr. Süveges Ildikó szemész
Dr. Szekanecz Zoltán reumatológus, belgyógyász, immunológus
Dr. Szijártó Attila sebész
Tamásné Dr. Németh Ágnes gyógyszerhatástan szakgyógyszerész
Dr. Tenke Péter urológus
Dr. Tóth Judit jogász
Dr. Vértes András kardiológus
Dr. Vidnyánszky Zoltán biológus
Módszertani levelek
– BETEGKÁRTYA szakmai-etikai követelmények (2015.12.15.)
TEMPLATES:
Investigator Curriculum Vitae (2023.03.)
Site Suitability Form (2023.10.)
Ajánlások klinikai gyógyszervizsgálatot kezdeményezőknek
A bioszimiláris igazolására tervezett klinikai gyógyszervizsgálatok szakmai-etikai értékelése (2022.11.28.)
Ajánlás a klinikai vizsgálatok betegtájékoztatóinak adatvédelmi követelményeiről a GDPR kapcsán (2023.03.14.)
TEMPLATES:
Investigator Curriculum Vitae (2023.03.)
Site Suitability Form (2023.10.)
Tájékoztatás a Gyógyszerrendelet alapján beadandó vizsgálati kérelmek benyújtóinak
National requirements for initial submissions / substatnital modifications
Originális vizsgálati kérelmekre és lényeges módosításokra vonatkozó nemzeti követelmények
The list of required documentation and information is set out in Annex I. of REGULATION (EU) No 536/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC.
In particular:
The application dossier for an application limited to Part I of the assessment report referred to in Article 11 shall be limited to sections B to J and Q of Annex I.
National requirements related to Part I:
Protocol synopsis in Hungarian language preferably a certified translation of the original protocol synopsis (not to be confused with trial summary for lay people);
The application dossier for an application limited to Part II of the assessment report referred to in Article 11 and the application dossier for an application referred to in Article 14 shall be limited to sections K to R of Annex I.
National requirements related to Part II of the application dossier in particular include
CV of PIs and co-investigators according to the templates proposed by CTEG as modified for Hungary and available on our homepage;
Site suitability templates according to the templates proposed by CTEG as modified for Hungary and available on our homepage;
Patient emergency card as addenda to the (main) ICF according to the specifications available on our homepage;
All recruitment material in their final forms in Hungarian language;
Protocol signature pages signed by PIs and Co-investigators;
A list in Hungarian language of Part II documents submitted in order to help the lay members of the Ethics Committee for Clinical Pharmacology to evaluate the submission.
In case both Part I and Part II application dossiers are submitted at the same time, all documents listed above should be provided for evaluation.
List of modified documents (including all newly created documents, e.g. templates for new PI/new site etc.) should be included in the cover letter in Hungarian language in case of substantial modifications. For accurate identification of new and/or updated documents, their version number and date of issue must also be provided. New documents must be submitted in both clean and change tracking formats. Conformity to all requirements will be thoroughly checked for applications submitted after 01.07.2023.
AZ ENGEDÉLY IRÁNTI KÉRELEMHEZ SZÜKSÉGES DOKUMENTÁCIÓ AZ EURÓPAI PARLAMENT ÉS A TANÁCS 536/2014/EU RENDELETE (2014. április 16.) az emberi felhasználásra szánt gyógyszerek klinikai vizsgálatairól és a 2001/20/EK irányelv hatályon kívül helyezéséről I. Melléklete szerint.
Részletezve:
Az értékelő jelentés I. részében szereplő szempontokra korlátozódó engedélyezéshez szükséges dokumentáció I. Melléklet B–J. és Q. szakaszára korlátozódik.
Az értékelő jelentés I. részéhez kapcsolódó magyar tagállami követelmény:
Magyar nyelvű protokoll szinopszis az eredeti protocol synopsis lehetőleg hivatalos fordítása, nem tévesztendő össze a laikusok számára készített vizsgálati összefoglalóval
Az értékelő jelentés II. részében szereplő szempontokra korlátozódó engedélyezéshez szükséges dokumentáció az I. Melléklet K–R. szakaszára korlátozódik.
Az értékelő jelentés II. részéhez kapcsolódó magyar tagállami követelmények:
A vizsgálatvezetők és a társvizsgálók szakmai önéletrajzai a honlapunkon megtalálható, a Magyarországi kívánalmakhoz igazított CTEG templát szerint;
A vizsgálatvezetők és a társvizsgálók által aláírt protocol signature oldalalak;
A vizsgálóhelyek megfelelőségét igazoló dokumentumok, a honlapunkon megtalálható, a Magyarországi kívánalmakhoz igazított CTEG templát szerint;
A fő tájékozott belegyezési dokumentum mellékleteként felcsatolt betegkártya, a honlapunkon található kívánalmaknak megfelelően elkészítve;
Minden toborzó dokumentum végleges formában, magyar nyelven;
Az értékelő jelentés II. részéhez benyújtott dokumentumok listája, melynek célja a KFEB nem orvos tagjai számára történő segítségnyújtás a beterjesztett dokumentumok értékeléséhez.
Amennyiben a vizsgálati kérelem I. és a II. részét egyidejűleg nyújtják be, minden fent felsorolt dokumentumot egyszerre kell a Bizottság rendelkezésére bocsájtani.
A lényeges módosítási kérelmek estében feltétlenül szükséges a módosított dokumentumok (beleértve az új dokumentumokat, pl. új vizsgálóhely/vizsgálatvezető stb. adatait) felsorolása a magyar nyelvű kísérőlevélben. Az új, illetve frissített dokumentumok pontos azonosítása céljából azok verziószámát és kiadásuk dátumát is meg kell adni. Az új dokumentumokat tiszta és változás követő formában is be kell nyújtani.
A fenti követelményeknek való megfelelést a Bizottság szigorúan ellenőrizni fogja a 2023.07.01. után benyújtott kérelmek esetében.
TEMPLATES:
Investigator Curriculum Vitae (2023.03.)
Site Suitability Form (2023.10.)
Part II kérelmek értékelésére vonatkozó kérdések és válaszok
Q1: Melyek az ETT KFEB tevékenységét meghatározó legfontosabb jogszabályok?
A1: Felhívjuk a benyújtók figyelmét a következő jogszabályokban foglaltakra:
• Az egészségügyről szóló 1997. évi CLIV. törvény (Egészségügyi törvény) (különösen a VIII. és IX. fejezete)
• Az emberi alkalmazásra kerülő gyógyszerekről és egyéb, a gyógyszerpiacot szabályozó törvények módosításáról szóló 2005. évi XCV. törvény (Gyógyszertörvény)
• A humángenetikai adatok védelméről, a humángenetikai vizsgálatok és kutatások, valamint a biobankok működésének szabályairól szóló 2008. évi XXI. törvény (Genetikai törvény)
• Az emberi felhasználásra szánt gyógyszerek klinikai vizsgálatairól és a 2001/20/EK irányelv hatályon kívül helyezéséről szóló, 2014. április 16-i 536/2014/EU európai parlamenti és tanácsi rendelet (CTR)
• A természetes személyeknek a személyes adatok kezelése tekintetében történő védelméről és az ilyen adatok szabad áramlásáról, valamint a 95/46/EK rendelet hatályon kívül helyezéséről (általános adatvédelmi rendelet) szóló, 2016. április 27-i (EU) 2016/679 európai parlamenti és tanácsi rendelet (GDPR)
• Az információs önrendelkezési jogról és az információszabadságról szóló 2011. évi CXII. törvény
• Az egészségügyi és a hozzájuk kapcsolódó személyes adatok kezeléséről és védelméről szóló 1997. évi XLVII. törvény
Fentieken túlmenően az ETT KFEB honlapján található információk ismerete segíti a benyújtott kérelmek gyors elfogadását.
Q2: Mi lehet az ICF címe és hány dokumentumból kell állnia?
A2: Tájékoztatáson alapuló beleegyező nyilatkozat, egyetlen dokumentum. Kivételt képez(nek) a genetikai tájékoztató(k), és beleegyező nyilatkozat(ok), amely(ek)nek különálló dokumentum(ok)nak kell lenniük a Genetikai törvény előírásai miatt. Minden dokumentumhoz egyetlen aláírás sorozat tartozik. Amennyiben a dokumentum címzettje nem tud írni/olvasni és ezért szóban ismertetik vele a dokumentumot, illetve szóban adja a beleegyezését, úgy két tanúnak is alá kell írnia a dokumentumot. A tanúknak meg kell adni a lakcímét (elérhetőségét) is.
Q3: Mit kell tartalmaznia a Tájékoztatáson alapuló Beleegyező nyilatkozatnak?
A3: A vonatkozó jogszabályok és az ETT KFEB döntési gyakorlatának megfelelően a Tájékoztatáson alapuló Beleegyező nyilatkozatnak a következőket kell tartalmaznia:
1. a klinikai vizsgálat azonosító adatait (EUCT szám, protokollszám és a vizsgálat címe is fontos),
2. annak az egészségügyi szolgáltatónak a megnevezését, ahol a klinikai vizsgálatot végezni kívánják,
3. a klinikai vizsgálat vezetőjének, illetve a tájékoztatást végző személynek a nevét, beosztását, munkaköre megnevezését,
4. a vizsgálati alany személyazonosító adatait (nevét, születési helyét és idejét), korlátozottan cselekvőképes kiskorú és cselekvőképességében az egészségügyi ellátással összefüggő jogok gyakorlása tekintetében részlegesen korlátozott vagy cselekvőképtelen vizsgálati alany esetén az Eütv. 16. §-a szerinti nyilatkozattételre jogosult személy (a továbbiakban: nyilatkozattételre jogosult személy) azonosító adatait is,
Ugyanakkor a beteg vizsgálati kódszámát nem tartalmazhatja a dokumentum.
5. a betegkártya kiállításáról szóló tájékoztatást,
6. a betegkártyán szerepelő adatokat, ezek kezelésére vonatkozó szabályokat,
7. az arra vonatkozó figyelemfelhívást, hogy a vizsgálati alany a betegkártyát saját érdekében a vizsgálat során mindig tartsa magánál,
8. tájékoztatást arra vonatkozóan, hogy a betegkártyán a vizsgálati alany neve és kódszáma csak betegbiztonsági okból szerepelhet együtt,
9. a vizsgálati alany adatainak kezelésére, az azokhoz való hozzáférésére vonatkozó szabályokat,
10. a klinikai vizsgálat kutatási jellegére való utalást, a klinikai vizsgálat célját, várható időtartamát, a bevonni kívánt személyek számát, a klinikai vizsgálat menetét, a tervezett beavatkozások jellegét, gyakoriságát,
11. a vizsgálati alany rendelkezésére álló egyéb, elfogadott kezelési lehetőségeket, valamint tájékoztatást arra vonatkozóan, hogy a klinikai vizsgálat a már megkezdett kezelésének megszakítását jelentheti, és a megkezdett kezelés megszakításának milyen következményei lehetnek a vizsgálati alany számára,
12. a lehetséges és a várható következmények, kockázatok és kellemetlenségek részletes leírását, valamint az arra való utalást, hogy a klinikai vizsgálat során olyan nemkívánatos események is felléphetnek, amelyek előre nem láthatóak,
13. az ésszerűen várható előnyök leírását, vagy ha a vizsgálati alany számára előny a klinikai vizsgálatból nem várható, ennek a ténynek a közlését,
14. placebo alkalmazásakor részletes tájékoztatást a placebo alkalmazásának lényegéről és arról, hogy a vizsgálati alany milyen valószínűséggel kerülhet placebo csoportba,
15. a vizsgálati gyógyszer rövid hatástani ismertetését,
16. azt, hogy a vizsgálat befejezése után a vizsgálati alany – amennyiben szükséges – milyen további egészségügyi ellátásban részesül,
17. arra vonatkozó figyelemfelhívást, hogy a vizsgálati alany – korlátozottan cselekvőképes kiskorú és cselekvőképességében az egészségügyi ellátással összefüggő jogok gyakorlása tekintetében részlegesen korlátozott vagy cselekvőképtelen vizsgálati alany esetén a nyilatkozattételre jogosult személy – a klinikai vizsgálatban történő részvételre vonatkozó beleegyezése önkéntes és befolyásolástól mentes, az bármikor akár szóban, akár írásban indokolás nélkül visszavonható anélkül, hogy ebből a vizsgálati alanynak hátránya származna,
18. a vizsgálati alany számára a klinikai vizsgálattal összefüggő kár bekövetkezése, illetve személyiségi jog megsértése esetén nyújtandó kezelésre, kár megtérítésére, illetve sérelemdíj megfizetésére és kártalanításra [GyT. 21. § (1) bekezdés] és az ezek igénybevételének módjára vonatkozó tájékoztatást, továbbá annak a személynek és szervezetnek a nevét és magyarországi elérhetőségét, akihez vagy amelyhez a vizsgálati alany a kár bekövetkezésekor fordulhat. Az egészségkárosodással kapcsolatos tájékoztatásnak ki kell térnie a kártérítésre, kártalanításra és sérelemdíjra is. A vizsgálat alanyát teljeskörű sérelemdíj és teljeskörű kártérítés illeti meg (2005. évi XCV. Gyógyszertörvény 21.§ (1) bekezdés). Amennyiben a vizsgálati alany egészségkárosodást szenved, az engedélyező hatóság által jóváhagyott terv szerint végzett vizsgálat esetén, akkor a kezelésének a költségeit a Szponzor (Megbízó), illetve annak biztosítója fedezi a Gyógyszertörvény 21§ (1) bekezdés a) pontja, és a Gyógyszertörvény 3.§ (4) bekezdése alapján. Az egészségkárosodást szenvedett vizsgálati alanynak az eseményt a felelősség vélelmezése nélkül a vizsgálatvezető és a betegtájékoztatóban megadott biztosító felé a lehető leghamarabb jeleznie kell.
19. a GyT. 3. § (4) bekezdése szerinti felelősségbiztosításban szereplő biztosító megnevezését, magyarországi elérhetőségét és kapcsolattartási adatait, ideértve a biztosító kapcsolattartójának megnevezését és telefonszámát.
20. a vizsgálati alany számára járó költségtérítést, ha van ilyen, kiemelve, hogy csak igazolt költségeket lehet megtéríteni. Pontosan fel kell sorolni, hogy melyek azok a költség típusok, amelyeket a szponzor meg kíván téríteni. A példálózó megfogalmazás, vagy feltételes mód használata („megtérítheti”) nem megfelelő. Amennyiben a költségtérítésben a szponzor külső szolgáltatót vesz igénybe, a szolgáltató és a beteg közötti szerződés lényeges elemeit, különös tekintettel az adatvédelmi kérdésekre és az esetleges szolgáltatói díjakra, a betegtájékoztató dokumentumnak tartalmaznia kell és azokba a betegnek bele kell egyeznie. Amennyiben a költségtérítés a szponzortól független szolgáltató segítségével történik, fel kell sorolni, hogy a szolgáltató számára milyen adatokat ad át a vizsgálóhely. Ha az adatokat közvetlenül a beteg adja át a szolgáltatónak egy szerződésnek megfelelően, amelyet a beteg a szolgáltatóval köt, akkor ennek a szerződésnek a lényeges tartalmi elmeit a fel kell tüntetni a Tájékoztatáson alapuló Beleegyező nyilatkozatban.
21. a tájékoztatáson alapuló beleegyező nyilatkozat aláírásának dátumát,
22. a klinikai vizsgálat vezetőjének vagy a tájékoztatást adónak az aláírását,
23. a tájékoztatáson alapuló beleegyező nyilatkozatot tevő aláírását.
Q4: Milyen tájékoztatást kell tartalmaznia a Tájékoztatáson alapuló beleegyező nyilatkozatnak a vizsgálati alany személyes adatainak a kezelésére vonatkozóan?
A4:
1. Az adatvédelmi tájékoztatóban egyértelműnek kell lennie, hogy az adatkezelő személyes adatokat, kódolt vagy anonim adatokat kezel, és ezt következetesen kell alkalmazni az adatvédelmi tájékoztatóban. Kerülni kell a pontatlan, nem egyértelmű, illetve a felelősség elhárítását szolgáló kijelentéseket, mint pl. „valószínűleg”, „előfordulhat” vagy, hogy „nem tudja a megbízó a személyes adatok védelmét biztosítani”. A kezelt adatokat tételesen fel kell sorolni. A gyűjteni kívánt adatok között nem szerepelhet a „faji hovatartozás” megjelölés, helyette az „etnikai hovatartozás” fogadható el. Nem gyűjthetők a szexuális életre vonatkozó adatok, eltekintve a különleges, orvos szakmai indokoltságú esetektől.
2. A tájékoztatóban egyértelműen szerepelnie kell annak, hogy a vizsgálóhelyről a vizsgálati alanyok személyes és különleges adatai (így például az egészségügyi adatok vagy az etnikai származásra vonatkozó adat) csak kódolt vagy anonimizált formában továbbíthatók, továbbá, hogy kódolt adat esetén a kódkulcs nem továbbítható. Kivételes és a beteg érdekei alapján indokolt esetben együtt szerepelhetnek a beteg születési adatai és neve a vizsgálati kódszámával. Az adatokhoz való hozzáférés körében az adatvédelmi tájékoztatóban fel kell sorolni a betekintési, illetve hozzáférési joggal rendelkező személyeket, és kijelenteni, hogy ők is csak a vizsgálóhelyen tekinthetnek bele ezen adatokba, és az adatokról nem készíthetnek másolatot.
3. Ki kell térni az adattovábbítás tényére, az adattovábbítás és különösen a harmadik országba való adattovábbítás garanciáira, a címzettek pontos megnevezésére, valamint meg kell nevezni az adatkezelés körülményeit: az adatokat hogyan kódolják, mennyi ideig és hol tárolják, és ezeket hogyan használják fel a vizsgálattal kapcsolatban. Az adatok másodlagos felhasználása esetén (amennyiben nem anonim adatokról van szó, és az elsődleges felhasználáskor nem tájékoztatták a vizsgálati alanyt az adat további felhasználásának céljáról) a vizsgálati alany újbóli beleegyezése szükséges. Ezeket a követelményeket az olyan vizsgálatokra is alkalmazni kell, amikor pl. vér, illetve szövetminta vételére kerül sor a vizsgálat/alvizsgálat folyamán. Ezek vonatkoznak genetikai adatok továbbítására is. Ilyen esetekben a Genetikai törvényre mindenképp hivatkozni kell!
4. Az adatkezelési tájékoztatónak feltétlenül tartalmaznia kell az adatkezelés célját és az adatkezelés jogalapját, ami a klinikai gyógyszervizsgálatok esetében csak az érintett hozzájárulása lehet.
5. A vizsgálati alanyt tájékoztatni kell arról, hogy ha a rá vonatkozó adatkezelés során jogai sérülnek, akkor Magyarországon kihez fordulhat jogai érvényesítése érdekében, továbbá meg kell adni a Nemzeti Adatvédelmi és Információszabadság Hatóság (NAIH) postai címét és internetes elérhetőségét is.
Q5: Be kell nyújtani a Betegkártyát a Part II engedélyeztetési dokumentumai között?
A5: Igen. A Betegkártyát magyar nyelven, végleges formájában kell benyújtani. A CTIS-ben célszerűen az L2 dokumentumok között lehet benyújtani a betegkártyát.
Q6: Mit kell tartalmaznia a betegkártyának?
A6: A betegkártyán feltétlenül szerepeljen
1. a vizsgálatban résztvevő beteg neve, születési ideje,
2. a vizsgálat címe (protokoll száma),
3. a vizsgálati gyógyszer megnevezése, továbbá a vizsgálati gyógyszer hatásmódja (például kináz gátló, monoklonális ellenanyag stb.), amennyiben az elnevezésből ez nem derül ki,
4. a vizsgálatban szereplő indikációja,
5. ellenjavallata,
6. veszélyes interakciók,
7. a vizsgálóhely címe,
8. a vizsgálatot vezető orvos (vagy egy általa megjelölt másik orvos) neve, 24 órán át elérhető telefonszáma,
9. minden egyéb szükségesnek tartott információ, például a vizsgálatban alkalmazott szerek esetlegesen sürgősségi beavatkozást indokló mellékhatásai.
Q7: Milyen egyéb dokumentumokat kell még benyújtani a vizsgálati kérelem Part II részének magyarországi engedélyeztetéséhez, illetve meglévő engedély lényeges módosítási kérelméhez?
A7: A CTR Annex 1 K-R fejezeteiben felsoroltakon kívül be kell nyújtani
1. egy magyar nyelvű kísérőlevelet, mely pontos listát tartalmaz az engedélyeztetni kívánt dokumentumok megnevezésével, beleértve azok verziószámát és kibocsátási dátumát is,
2. a betegkártyát,
3. a vizsgálatvezetők által aláírt protokoll elfogadási nyilatkozatokat.
Q8: Kell-e ügyelni a nyelvhelyességre?
A8: Igen. Tekintettel arra, hogy az alapdokumentumok általában angol nyelven készülnek, a szó szerinti fordítás sok esetben egyenetlen színvonalú és sokszor zavaró a nem megfelelő igeragozás (pl. tegező/magázó formák keveredése). A nyelvi lektorálás hiánya néha értelemzavaró hibákhoz vezet. Magyarországon nem érvényes, pl. USA-beli jogszabályokra történő hivatkozás helyett a megfelelő magyar jogszabályokat kell idézni. Mindezen hibák kijavítását a Bizottság általában hiánypótlási felszólítás formájában kéri, ami az eljárás hosszát szükségtelenül nyújtja meg és odafigyeléssel elkerülhető lenne.
Q9: Mi a vizsgált hatóanyagot tartalmazó preparátum hivatalos magyar megnevezése?
A9: Vizsgálati gyógyszer a helyes megnevezés.
Q10: Mire kell a lényeges módosítási kérelmek esetében speciálisan ügyelni?
A10: A lényeges módosítások kísérőlevelében pontosan fel kell sorolni a módosítani kívánt dokumentumokat (verziószám és kibocsátási dátum megadásával), le kell írni a módosítás lényegét és indokait és be kell nyújtani a tiszta és változás követő formátumot is. Utóbbira természetesen nincsen szükség, ha az adott dokumentum a korábbiakban még egyáltalán nem került benyújtásra.
Az ETT KFEB által tárgyalt klinikai vizsgálati kérelmek (2022)
| Sorszám és cím |
|---|
| 1. AN OPEN-LABEL, MULTI-CENTRE STUDY TO EVALUATE THE LONG-TERM SAFETY AND TOLERABILITY OF REN001 IN SUBJECTS WITH PRIMARY MITOCHONDRIAL MYOPATHY (PMM) |
| 2. A Comparative, Randomized, Double-blind, 3-arm parallel, Phase III Study to Evaluate the Efficacy and Safety of a Fixed Dose Combination of Nefopam/Paracetamol (tablet) Taken Orally in Moderate to Severe Pain After Impacted Third Molar Extraction |
| 3. A 6-month multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety and PK/PD of an age-and body weightadjusted oral finerenone regimen, in addition to an ACEI or ARB, for the treatment of children, 6 months to <18 years of age, with chronic kidney disease and proteinuria |
| 4. A Phase 2B prospective, blinded, randomized, placebo controlled, international multicenter study to assess restoration of coronary artery blood flow and resolution of ST segment deviation after a single subcutaneous injection of RUC-4 in subjects with ST-elevation myocardial infarction presenting in the ambulance (pre-hospital setting) |
| 5. A Phase 3, Randomized, Double-Blind Study of MK-7684A in Combination with Etoposide and Platinum Followed by MK-7684A vs Atezolizumab in Combination with Etoposide and Platinum Followed by Atezolizumab for the First-Line Treatment of Participants with Extensive-Stage Small Cell Lung Cancer |
| 6. An Open-Label, Multicenter, Extension Study for Subjects Who Participated in Prior Clinical Studies of ASTX727 (Standard Dose), With a Food Effect Substudy at Select Study Centers |
| 7. A Phase 1/2, Multi-Center, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Evidence of Antitumor Activity of JAB-21822 Monotherapy and Combination Therapy in Adult Patients with Advanced Solid Tumors Harboring KRAS G12C Mutation |
| 8. Randomised, double-blind, placebo-controlled and parallel dose group trial to investigate efficacy and safety of multiple doses of oral BI 690517 over 14 weeks, alone and in combination with empagliflozin, in patients with diabetic and non-diabetic chronic kidney disease |
| 9. Evaluation of the bioequivalence of two products containing aripiprazole: Aripiprazole LAI powder and solvent for prolonged-release suspension for intramuscular injection, 400mg, Greece (Test) vs. ABILIFY MAINTENA® (aripiprazole) for extended-release injectable suspension, for intramuscular use, 400mg, USA (Reference). A multicentric, multi-national, open-label, randomized, multiple dose, two-period, crossover pharmacokinetic study in schizophrenic patients |
| 10. A Phase 2 Open-label, Long-term Extension Safety Study of Brazikumab in Participants with Moderately to Severely Active Ulcerative Colitis (EXPEDITION OLE) |
| 11. READY 2: A PHASE 3, OPEN LABEL, SINGLE ARM STUDY ON THE USE OF CUSA-081 FOR DYSFUNCTIONAL CENTRAL VENOUS ACCESS DEVICES (CVADs) |
| 12. A Randomized, Open Label Phase 3 Study Evaluating Safety and Efficacy of Venetoclax in combination with Azacitidine after allogeneic Stem Cell Transplantation in Subjects with Acute Myeloid Leukemia (AML) (VIALE-T) |
| 13. A randomized, double-blind, double-dummy, parallel-group study, comparing the efficacy and safety of remibrutinib 100 mg b.i.d. versus teriflunomide 14 mg q.d. in participants with relapsing multiple sclerosis, followed by extended treatment with open-label remibrutinib |
| 14. OPEN-LABEL INDUCTION AND MAINTENANCE STUDY OF ORAL CP-690,550 (TOFACITINIB) IN CHILDREN WITH MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS |
| 15. A phase Ib/II open label dose confirmation, proof of concept study of siremadlin in combination with venetoclax plus azacitidine in unfit adult AML participants who responded sub-optimally to first-line venetoclax plus azacitidine treatment and in participants with newly diagnosed unfit AML presenting with high-risk clinical features. |
| 16. A randomised, double-blind, placebo-controlled, parallel group, dosefinding study evaluating efficacy, safety and tolerability of BI 1291583 qd over at least 24 weeks in patients with bronchiectasis |
| 17. A MULTICENTER, OPEN-LABEL STUDY TO EVALUATE THE SAFETY AND EFFICACY OF ONCE DAILY ORAL VADADUSTAT FOR THE TREATMENT OF PEDIATRIC SUBJECTS WITH ANEMIA OF CHRONIC KIDNEY DISEASE AFTER CONVERSION FROM AN ERYTHROPOIESIS-STIMULATING AGENT |
| 18. A Randomized, Double-blind, Placebo-controlled Phase 3 Study of Tamibarotene Plus Azacitidine Versus Placebo Plus Azacitidine in Newly Diagnosed Adult Patients Selected for RARA-positive Higher-risk Myelodysplastic Syndrome |
| 19. A MULTICENTER, OPEN-LABEL STUDY TO EVALUATE THE SAFETY AND EFFICACY OF ONCE DAILY ORAL VADADUSTAT FOR THE TREATMENT OF PEDIATRIC SUBJECTS WITH ANEMIA OF CHRONIC KIDNEY DISEASE NAIVE TO ERYTHROPOIESIS-STIMULATING AGENTS |
| 20. AN OPEN-LABEL, MULTI-CENTRE STUDY TO EVALUATE THE LONG-TERM SAFETY AND TOLERABILITY OF REN001 IN SUBJECTS WITH PRIMARY MITOCHONDRIAL MYOPATHY (PMM) |
| 21. A Randomised, Double-Blind, Placebo-controlled study of ALPN-101 in Systemic Lupus Erythematosus |
| 22. A Phase 3 Open-Label, Randomized, Controlled, Global Study of Telisotuzumab Vedotin (ABBV-399) Versus Docetaxel in Subjects with Previously Treated c-Met Overexpressing, EGFR Wildtype, Locally Advanced/Metastatic Non-Squamous Non-Small Cell Lung Cancer |
| 23. Master protocol of two randomized, double blind, placebo-controlled, multicenter, parallel group studies to evaluate the efficacy and safety of dupilumab in adult patients with chronic pruritus of unknown origin (CPUO) |
| 24. Gadopiclenol Pharmacokinetics, Safety and Efficacy in Pediatric Patients < 2 Years of Age Undergoing Contrast-enhanced MRI P/0145/2019 |
| 25. A PHASE IIIB, MULTICENTER, RANDOMIZED, VISUAL ASSESSOR-MASKED STUDY OF THE EFFECTIVENESS AND SAFETY OF A 36-WEEK REFILL REGIMEN FOR THE PORT DELIVERY SYSTEM WITH RANIBIZUMAB VS AFLIBERCEPT TREAT & EXTEND IN SUBJECTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION (DIAGRID) |
| 26. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Magrolimab versus Placebo in Combination with Venetoclax and Azacitidine in Newly Diagnosed, Previously Untreated Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy |
| 27. A Phase 3, Prospective, Open-Label, Multisite, Extension of Phase 3 Studies To Assess the Long-Term Safety and Tolerability of Soticlestat as Adjunctive Therapy in Subjects With Dravet Syndrome or Lennox-Gastaut Syndrome (ENDYMION 2). |
| 28. A Phase 3 Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Daily Subcutaneous Injections of Elamipretide in Subjects with Primary Mitochondrial Disease Resulting from Pathogenic Nuclear DNA Mutations (nPMD) |
| 29. A Randomized, Phase 3, Double Masked, Parallel Group, Multicenter Study to Compare the Efficacy and Safety of ALT L9 Versus Eylea® in Patients With Neovascular Age Related Macular Degeneration (ALTERA) |
| 30. A PHASE II, MULTICENTER, RANDOMIZED, DOUBLE MASKED, ACTIVE COMPARATORCONTROLLED STUDY TO INVESTIGATE THE EFFICACY, SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF RO7200220 ADMINISTERED INTRAVITREALLY IN PATIENTS WITH DIABETIC MACULAR EDEMA |
| 31. A PHASE II, MULTICENTER, RANDOMIZED, DOUBLE MASKED, ACTIVE COMPARATORCONTROLLED STUDY TO INVESTIGATE THE EFFICACY, SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF RO7200220 IN COMBINATION WITH RANIBIZUMB ADMINISTERED INTRAVITREALLY IN PATIENTS WITH DIABETIC MACULAR EDEMA |
| 32. A PHASE III, RANDOMIZED, OPEN-LABEL, MULTICENTER STUDY OF LURBINECTEDIN IN COMBINATION WITH ATEZOLIZUMAB COMPARED WITH ATEZOLIZUMAB AS MAINTENANCE THERAPY IN PARTICIPANTS WITH EXTENSIVE-STAGE SMALL-CELL LUNG CANCER (ES-SCLC) FOLLOWING FIRST-LINE INDUCTION THERAPY WITH CARBOPLATIN, ETOPOSIDE AND ATEZOLIZUMAB |
| 33. AVELUMAB MASTER PROTOCOL: AN OPEN-LABEL CONTINUATION STUDY FOR PARTICIPANTS CONTINUING FROM PFIZER SPONSORED AVELUMAB CLINICAL STUDIES |
| 34. AN INTERVENTIONAL PHASE 2, OPEN-LABEL, ONE-ARM, MULTI-CENTER STUDY TO EVALUATE SAFETY AND EFFICACY OF PF-06835375 IN ADULT PARTICIPANTS WITH MODERATE TO SEVERE PRIMARY IMMUNE THROMBOCYTOPENIA |
| 35. Phase 1 Pharmacokinetics and Safety Study of Oral Soticlestat in Participants with Moderate or Severe Hepatic Impairment and Normal Hepatic Function |
| 36. AN OPEN LABEL, LONG-TERM EXTENSION STUDY TO INVESTIGATE THE SAFETY OF PF-06823859 ADMINISTERED TO ADULT PARTICIPANTS >= 18 AND =< 80 WITH ACTIVE DERMATOMYOSITIS. |
| 37. A Phase 1b study in patients with acromegaly or functioning gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to characterize the pharmacokinetics, pharmacodynamics, safety and tolerability of Debio 4126, a 12-week prolonged-release octreotide formulation |
| 38. A Phase 1, first-in-human, three-part, randomized, double-blind, placebocontrolled study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SAR443726 in healthy adult participants and in adult participants with moderate-to-severe atopic dermatitis. |
| 39. postMONARCH: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Compare the Efficacy of Abemaciclib plus Fulvestrant to Placebo plus Fulvestrant in Participants with HR+, HER2-, Advanced or Metastatic Breast Cancer Following Progression on a CDK4 & 6 Inhibitor and Endocrine Therapy |
| 40. A Three-Part, Phase 1, Randomized, Controlled, Dose-Escalation Study of INT-787 Following Single or Multiple Dose Administration in Healthy Subjects |
| 41. A Multicenter, Double-blind, Randomized Phase 3 Study to Compare the Efficacy and Safety of Belzutifan (MK-6482) Plus Pembrolizumab (MK-3475) Versus Placebo Plus Pembrolizumab, in the Adjuvant Treatment of Clear Cell Renal Cell Carcinoma (ccRCC) Post Nephrectomy (MK-6482-022) |
| 42. A randomized double blind placebo controlled multicenter study to assess the efficacy and tolerability of tolperisone as add on treatment with standardized NSAID of acute non specific low back pain. |
| 43. Phase I /II, open label, dose escalation part (phase I) followed by non-comparative expansion part (phase II), multi-centre study, evaluating safety, pharmacokinetics and efficacy of S65487, a Bcl2 inhibitor combined with azacitidine in adult patients with previously untreated acute myeloid leukemia not eligible for intensive treatment |
| 44. A Phase I, Randomised, Open-Label, Single-Dose, Two-Treatment, Two-Way Crossover, Two-Stage Study to Evaluate the Bioequivalence of Onivyde (Irinotecan Liposome Injection) Manufactured at Two Different Sites Administered in Combination with Anti-Cancer Agents in Adult Participants with Metastatic Pancreatic Adenocarcinoma |
| 45. A Randomized, Controlled, Multicenter, Open-label Study to Investigate the Efficacy and Safety of Adding Apalutamide to Radiotherapy and LHRH Agonist in High-Risk Patients with PSMA-PET-Positive Hormone-Sensitive Prostate Cancer, with an Observational Follow-up of PSMA-PET-Negative Patients. |
| 46. Comparison of the preventive painkiller effect of etoricoxib and celecoxib after M3M surgery: A randomized, double-masked clinical trial |
| 47. A LONG-TERM, OPEN-LABEL FOLLOW-UP STUDY OF TOFACITINIB FOR TREATMENT OF JUVENILE IDIOPATHIC ARTHRITIS (JIA) |
| 48. A Double-Blinded, Placebo-controlled, Multi-center Randomized, Phase 3 Study to Evaluate the Efficacy and Safety of ORMD-0801 in Subjects with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Diet Control Alone or on Diet Control and Glucose-lowering Agents as Monotherapy. |
| 49. A Randomized, Double-Blind, Placebo controlled Study Assessing the Longterm Effect of Dupilumab on Prevention of Lung Function Decline in Patients with Uncontrolled Moderate to Severe Asthma |
| 50. An Open-Label, Multicenter, Phase 2 Study of the Safety and Efficacy of KRT-232 in Subjects with Relapsed or Refractory Small Cell Lung Cancer (SCLC) |
| 51. A Double Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of PRA023 in Subjects with Systemic Sclerosis Associated with Interstitial Lung Disease (SSc-ILD) |
| 52. A Phase III, Multicentre, Randomized, Double-blind, Chronic-dosing, Parallel-group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Two Dose Regimens of MEDI3506 in Participants with Symptomatic Chronic Obstructive Pulmonary Disease (COPD) with a History of COPD Exacerbations (Oberon) |
| 53. A Randomized, Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Paltusotine in Subjects with Non-pharmacologically Treated Acromegaly |
| 54. LuCa-MERIT-1: First-in-human, open label, Phase I dose confirmation trial evaluating the safety, tolerability and preliminary efficacy of BNT116 alone and in combinations in patients with advanced non-small cell lung cancer. |
| 55. A randomized, double-blind, parallel group, placebo-controlled, multicenter Phase 3 trial to evaluate the efficacy, safety and tolerability of ianalumab on top of standard-of-care therapy in patients with active lupus nephritis (SIRIUS-LN) |
| 56. A Phase 2/3, Randomized, Double-Blinded, Placebo-Controlled, Parallel- Group Study to Investigate the Efficacy and Safety of Efgartigimod PH20 SC in Adult Participants With Bullous Pemphigoid |
| 57. A randomized, multicenter, double-blind, Phase 3 study of amcenestrant (SAR439859) versus tamoxifen for the treatment of patients with hormone receptor-positive, human epidermal growth factor 2-negative or positive, stage IIB-III breast cancer who have discontinued adjuvant aromatase inhibitor therapy due to treatment-related toxicity |
| 58. A Phase III, Randomized, Doubleblind, Placebo-controlled, Multicentre, International Study of Durvalumab plus Domvanalimab (AB154) in Participants with Locally Advanced (Stage III), Unresectable Nonsmall Cell Lung Cancer Whose Disease has not Progressed Following Definitive Platinumbased Concurrent Chemoradiation Therapy (PACIFIC-8) |
| 59. A Phase 3, Randomized, Double-blind, Placebo-controlled Multicenter Study to Evaluate the Efficacy and Safety of Pegcetacoplan in Patients with Cold Agglutinin Disease (CAD) |
| 60. MAGNETISMM-7 A RANDOMIZED, 2-ARM, PHASE 3 STUDY OF ELRANATAMAB (PF-06863135) VERSUS LENALIDOMIDE IN PATIENTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA WHO ARE MINIMAL RESIDUAL DISEASE POSITIVE AFTER UNDERGOING AUTOLOGOUS STEM-CELL TRANSPLANTATION |
| 61. Obicetrapib and Cardiovascular Outcomes: A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With Atherosclerotic Cardiovascular Disease (ASCVD) Who are Not Adequately Controlled Despite Maximally Tolerated Lipid-Modifying Therapies |
| 62. A phase III, randomized, double-masked, placebo controlled, parallel-group, multicenter study of the safety and efficacy of OT-101 (Atropine 0.01%) in treating the progression of myopia in pediatric subjects |
| 63. A Randomized, Double-masked, Parallel-group, Multicenter Clinical Study to Evaluate the Efficacy and Safety of AVT06 Compared with EU-Eylea® in Subjects with Neovascular (wet) Age related Macular Degeneration (ALVOEYE) |
| 64. A PHASE 3, RANDOMIZED, PARTIALLY DOUBLE-BLIND TRIAL TO EVALUATE THE SAFETY AND IMMUNOGENICITY OF 20-VALENT PNEUMOCOCCAL CONJUGATE VACCINE IN HEALTHY TODDLERS 12 THROUGH 23 MONTHS OF AGE WITH 2 PRIOR INFANT DOSES OF PREVENAR 13 |
| 65. IOCYTE AMI-3: A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Intravenous FDY-5301 in Patients with an Anterior ST-Elevation Myocardial Infarction |
| 66. A Phase I, Open-Label Study to Evaluate the Pharmacokinetics of Tezepelumab in Children >= 5 to 11 Years of Age with Mild, Moderate, or Severe Asthma (TRAILHEAD) |
| 67. A Phase 3, Multicenter, Randomized, Parallel-Design, Open-Label Trial to Evaluate the Efficacy and Safety of LY3209590 Compared with Insulin Degludec in Participants with Type 2 Diabetes Currently Treated with Basal Insulin (QWINT-3) |
| 68. A Phase 2 Trial of MRTX849 Monotherapy and in Combination with Pembrolizumab in Patients with Advanced Non-Small Cell Lung Cancer with KRAS G12C Mutation |
| 69. A Phase 3, Multi-Center, Randomized, Double-blind, Placebo-controlled Trial to Evaluate the Efficacy and Safety of CK-3773274 in Adults with Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction |
| 70. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Guselkumab Subcutaneous Induction Therapy in Participants with Moderately to Severely Active Crohn’s Disease |
| 71. A Phase III Double-Blind, Randomised, Placebo-Controlled Study Assessing the Efficacy and Safety of Capivasertib + Docetaxel Versus Placebo + Docetaxel as Treatment for Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC) (CAPItello-280) |
| 72. A randomised, openlabel, multi-centre, two-arm Phase 3 study comparing futuximab/modotuximab in combination with trifluridine/tipiracil to trifluridine/tipiracil single agent with a Safety Lead-In part in participants with KRAS/NRAS and BRAF wild type metastatic colorectal cancer previously treated with standard treatment and anti-EGFR therapy (COLSTAR) |
| 73. A PHASE 2/3, INTERVENTIONAL SAFETY, PHARMACOKINETICS, AND EFFICACY, OPEN-LABEL, MULTI-CENTER, SINGLE-ARM STUDY TO INVESTIGATE ORALLY ADMINISTERED PF-07321332 (NIRMATRELVIR)/RITONAVIR IN NONHOSPITALIZED SYMPTOMATIC PEDIATRIC PARTICIPANTS WITH COVID-19 WHO ARE AT RISK OF PROGRESSION TO SEVERE DISEASE |
| 74. A Phase 3, Randomized, Double-blind, Controlled Study Evaluating the Efficacy and Safety of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Who Are Heterozygous for F508del and a Minimal Function Mutation (F/MF) |
| 75. A Phase 3, Randomized, Double-blind, Controlled Study Evaluating the Efficacy and Safety of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Who Are Homozygous for F508del, Heterozygous for F508del and a Gating (F/G) or Residual Function (F/RF) Mutation, or Have At Least 1 Other Triple Combination Responsive CFTR Mutation and No F508del Mutation |
| 76. A randomized, double-blind, placebo-controlled, parallel-group, multicenter Phase 3 study to investigate the efficacy and safety of FInerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease |
| 77. A Randomized, Double-blind, Placebo-controlled, Dose Ranging Phase 2 Study to Evaluate the Efficacy and Safety of RIST4721 in Subjects with Palmoplantar Pustulosis |
| 78. A non-randomized, open-label, 2-part, parallel-cohort trial to evaluate 1) pharmacokinetics, safety, and tolerability of a single dose of BI 456906 in patients with cirrhosis and varying degrees of hepatic impairment relative to healthy subjects and 2) safety and tolerability of multiple doses of BI 456906 in patients with overweight/obesity with cirrhosis and varying degrees of hepatic impairment relative to patients with overweight/obesity without cirrhosis/hepatic impairment |
| 79. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 2b Dose-Ranging Study to Evaluate the Efficacy and Safety of Orismilast in Adults With Moderate to Severe Atopic Dermatitis |
| 80. A phase III randomized, controlled, open-label, multicenter, global study of capmatinib in combination with osimertinib versus platinum – pemetrexed based doublet chemotherapy in patients with locally advanced or metastatic NSCLC harboring EGFR activating mutations who have progressed on prior EGFR-TKI therapy and whose tumors are T790M mutation negative and harbor MET amplification (GEOMETRY-E) |
| 81. A multicenter, randomized, open-label, blinded endpoint evaluation, phase 3 study comparing the effect of abelacimab relative to apixaban on venous thromboembolism (VTE) recurrence and bleeding in patients with cancer associated VTE |
| 82. A Subject-Blinded First-In-Human Phase I Study to Assess the Safety, Tolerability, and Pharmacokinetics of Single Ascending Doses of AST-004 As a Short Intravenous Infusion in Healthy Adult Subjects |
| 83. A Phase 2, Double-Masked, Placebo-Controlled, Dose Range Finding Study of Danicopan (ALXN2040) in Patients with Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration (AMD)” |
| 84. A multicenter, randomized, open-label, blinded endpoint evaluation, phase 3 study comparing the effect of abelacimab relative to dalteparin on venous thromboembolism (VTE) recurrence and bleeding in patients with gastrointestinal (GI)/genitourinary (GU) cancer associated VTE” |
| 85. A MULTICENTER, OPEN-LABEL PHASE IV STUDY TO EVALUATE OVERALL HEALTH, PHYSICAL ACTIVITY, AND JOINT OUTCOMES, IN PARTICIPANTS AGED ≥ 13 AND < 70 YEARS WITH SEVERE OR MODERATE HEMOPHILIA A WITHOUT FVIII INHIBITORS ON EMICIZUMAB PROPHYLAXIS” |
| 86. Adjuvant encorafenib & binimetinib vs. placebo in fully resected stage IIB/C BRAF V600E/K mutated melanoma: a randomized triple-blind phase III study in collaboration with the EORTC Melanoma Group” |
| 87. A Phase 3 Randomized Study Comparing Bortezomib, Lenalidomide and Dexamethasone (VRd) followed by Ciltacabtagene Autoleucel, a Chimeric Antigen Receptor T cell (CAR-T) Therapy Directed Against BCMA versus Bortezomib, Lenalidomide, and Dexamethasone (VRd) followed by Lenalidomide and Dexamethasone (Rd) Therapy in Participants with Newly Diagnosed Multiple Myeloma for Whom Hematopoietic Stem Cell Transplant is Not Planned as Initial Therapy” |
| 88. A Phase 3 Randomized, Open-label, Active-comparator Controlled Clinical Study of Pembrolizumab versus Platinum Doublet Chemotherapy in Participants With Mismatch Repair Deficient (dMMR) Advanced or Recurrent Endometrial Carcinoma in the First-line Setting (KEYNOTEC93/GOG-3064/ENGOT-en15)” |
| 89. A randomized, double-blind, double-dummy, parallel-group study, comparing the efficacy and safety of remibrutinib versus teriflunomide in participants with relapsing multiple sclerosis, followed by extended treatment with open-label remibrutinib” |
| 90. A Phase 3, Multicenter, Open-label, Basket, Long-term Extension Study of Ustekinumab in Pediatric Clinical Study Participants (2 to <18 Years of Age)” |
| 91. A randomized, double-blind, double-dummy, parallel-group study, comparing the efficacy and safety of remibrutinib versus teriflunomide in participants with relapsing multiple sclerosis, followed by extended treatment with open-label remibrutinib” |
| 92. A Randomized, Double-Blind, Placebo-Controlled, Phase 3, Three-way Crossover Trial to Evaluate the Efficacy and Safety of Two Dose Levels of KVD900, an Oral Plasma Kallikrein Inhibitor, for On-Demand Treatment of Angioedema Attacks in Adolescent and Adult Patients with Hereditary Angioedema Type I or II” |
| 93. A PHASE Ia/Ib DOSE-ESCALATION AND DOSE EXPANSION STUDY EVALUATING THE SAFETY, PHARMACOKINETICS, AND ACTIVITY OF GDC-6036 AS A SINGLE AGENT AND IN COMBINATION WITH OTHER ANTICANCER THERAPIES IN PATIENTS WITH ADVANCED OR METASTATIC SOLID TUMORS WITH A KRAS G12C MUTATION” |
| 94. A Phase I, Open-label, Parallel Group Study to Investigate Olaparib Safety and Tolerability, Efficacy and Pharmacokinetics in Paediatric Patients with Solid Tumours” |
| 95. A PHASE III, RANDOMIZED, OPEN-LABEL STUDY EVALUATING THE EFFICACY AND SAFETY OF GIREDESTRANT IN COMBINATION WITH PHESGO VERSUS PHESGO AFTER INDUCTION THERAPY WITH PHESGO+TAXANE IN PATIENTS WITH PREVIOUSLY UNTREATED HER2-POSITIVE, ESTROGEN RECEPTOR-POSITIVE LOCALLY-ADVANCED OR METASTATIC BREAST CANCER |
| 96. A PHASE III, RANDOMIZED, OPEN-LABEL STUDY EVALUATING THE EFFICACY AND SAFETY OF GIREDESTRANT IN COMBINATION WITH PHESGO VERSUS PHESGO AFTER INDUCTION THERAPY WITH PHESGO+TAXANE IN PATIENTS WITH PREVIOUSLY UNTREATED HER2-POSITIVE, ESTROGEN RECEPTOR-POSITIVE LOCALLY-ADVANCED OR METASTATIC BREAST CANCER |
| 97. A 96-Weeks, Prospective, Multicenter, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study to Compare Efficacy and Safety of Masitinib Dose Titration to 4.5 mg/kg/day versus Placebo in the Treatment of Patients with Primary Progressive or Secondary Progressive Multiple Sclerosis Without Relapse. |
| 98. A multicenter, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of apraglutide in adult subjects with short bowel syndrome and intestinal failure (SBS-IF) |
| 99. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study to Assess the Efficacy and Safety of Lumateperone as Adjunctive Therapy in the Treatment of Patients with Major Depressive Disorder |
| 100. A multi-center, randomized, double-blind, placebo controlled, parallelgroup Phase IIIb study evaluating the effect of inclisiran on atherosclerotic plaque progression assessed by coronary computed tomography angiography (CCTA) in participants with a diagnosis of non-obstructive coronary artery disease without previous cardiovascular events (VICTORION-PLAQUE) |
| 101. A Phase 2, Randomised, Double-Blind, Placebo and Active Comparator-Controlled Study to Assess Efficacy and Safety of Multiple Dose Levels of AZD5718 Given Orally Once Daily for Twelve Weeks in Adults with Moderate-to-Severe Uncontrolled Asthma |
| 102. A PHASE III, RANDOMIZED, OPEN-LABEL STUDY OF PRALSETINIB VERSUS STANDARD OF CARE FOR TREATMENT OF RET-MUTATED MEDULLARY THYROID CANCER |
| 103. An Open-Label, Multicenter, Phase 1b/2 Study of the Safety and Efficacy of TL-895 Combined with Ruxolitinib in Janus associated Kinase Inhibitor (JAKi) Treatment-Naive Myelofibrosis (MF) Subjects and Subjects with MF who have a Suboptimal Response to Ruxolitinib |
| 104. Tolerability and Safety of Inhaled Colistimethate Sodium Administered Once Daily Compared to Twice Daily Dosing in Adult and Adolescent Subjects with Cystic Fibrosis and Chronic Pseudomonas Aeruginosa Lung Infection (COPILOT) |
| 105. Part I. A Parallel-group (2-Arm), Randomized, Double-blind, 12-week Trial to Evaluate the Efficacy and Safety of MC2-25 Cream and MC2-25 Vehicle in Subjects with Chronic Kidney Disease-associated Pruritus (CKD-aP) |
| 106. Part II. A Parallel-group (2-Arm), Randomized, Double-blind, 12-week Trial to Evaluate the Efficacy and Safety of MC2-25 Cream and MC2-25 Vehicle in Subjects with Chronic Kidney Disease-associated Pruritus (CKD-aP) |
| 107. An open-label, randomized, Phase 3 clinical trial of IO102-IO103 in combination with pembrolizumab versus pembrolizumab alone in patients with previously untreated, unresectable, or metastatic (advanced) melanoma |
| 108. A multicenter, randomized, double-blind, parallel group, placebo controlled study to assess safety, tolerability, pharmacokinetics and pharmacodynamics of BI 764198 administered orally once daily for 12 weeks in patients with focal segmental glomerulosclerosis |
| 109. An Open-Label, Safety, Tolerability, and Proof-of-Concept Study of Oral BCX9930 Therapy in Subjects with Complement 3 Glomerulopathy, Immunoglobulin A Nephropathy, or Primary Membranous Nephropathy |
| 110. A randomized, double-blind, placebo controlled, 3-arm multicenter phase 3 study to assess the efficacy and safety of ianalumab in patients with active Sjögren’s syndrome (NEPTUNUS-2) |
| 111. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of ISIS 678354 Administered Subcutaneously to Patients with Severe Hypertriglyceridemia |
| 112. An adaptive, randomized, double-blind, dose exploration, parallel group, placebo-controlled, multicenter phase 2 trial to evaluate the efficacy, safety and tolerability of LNP023 in combination with standard-of-care with and without oral corticosteroids in patients with active lupus nephritis Class III-IV, +/- V |
| 113. A PHASE 2/3 STUDY TO EVALUATE THE EFFICACY AND SAFETY OF UNESBULIN IN UNRESECTABLE OR METASTATIC, RELAPSED OR REFRACTORY LEIOMYOSARCOMA |
| 114. A Multicenter, Single-arm, Open-label, Extension, Rollover Study To Evaluate The Long-term Safety And Efficacy Of Ocrelizumab In Patients With Multiple Sclerosis |
| 115. A Phase 3, Open-label, Randomised Study of Datopotamab Deruxtecan (Dato-DXd) Versus Investigator’s Choice of Chemotherapy in Patients who are not Candidates for PD-1/PD-L1 Inhibitor Therapy in First-line Locally Recurrent Inoperable or Metastatic Triple-negative Breast Cancer (TROPION-Breast02) |
| 116. A Phase 1b/2, Multicenter, Open-label Basket Study Evaluating the Safety and Efficacy of Bemarituzumab Monotherapy in Solid Tumors with FGFR2b Overexpression (FORTITUDE 301) |
| 117. A Multicenter, Double-blind, Randomized, Placebo-controlled Study to Evaluate the Efficacy and Safety of Nemolizumab in Subjects with Chronic Kidney Disease with Associated Severe Pruritus |
| 118. A Phase 3 Study of the Hepcidin Mimetic Rusfertide (PTG-300) in Patients with Polycythemia Vera |
| 119. A Phase 3, Single-Arm, Open-Label, Multicenter Study to Evaluate the Safety and Efficacy of Tafasitamab Plus Lenalidomide in Participants With Relapsed or Refractory Diffuse Large B-Cell Lymphoma |
| 120. A Double Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of PRA023 in Subjects with Systemic Sclerosis Associated with Interstitial Lung Disease (SSc-ILD) |
| 121. A Parallel Group Multiple Ascending Dose, Phase 1, Double-Blind, 5-Cohort Study to Investigate the Safety and Pharmacokinetics of NTRX 07 in Male or Female Healthy Volunteers and Patients with Early Alzheimer’s Disease (AD), with an Exploratory Fed-Fasted Assessment |
| 122. A phase 3, multicenter, multinational, randomized, double-blind, doubledummy, active-comparator study to evaluate the efficacy and safety of venglustat in adult and pediatric patients with Gaucher disease Type 3 (GD3) who have reached therapeutic goals with Enzyme Replacement Therapy (ERT) |
| 123. Part I. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Clinical Study to Assess the Effectiveness of CRD-740 in Subjects with Chronic Heart Failure |
| 124. Part II A Phase 2, Randomized, Double-Blind, Placebo-Controlled Clinical Study to Assess the Effectiveness of CRD-740 in Subjects with Chronic Heart Failure |
| 125. A Phase 3, randomized, placebo-controlled, observer-blind, multi-country study to demonstrate the efficacy of a single dose and annual revaccination doses of GSK’s RSVPreF3 OA investigational vaccine in adults aged 60 years and above. |
| 126. MAJIC: A Phase III Prospective, Multicenter, Randomized, Open-Label Trial of Acalabrutinib plus Venetoclax versus Venetoclax plus Obinutuzumab in Previously Untreated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma |
| 127. KontRASt-02: A randomized, controlled, open label, phase III study evaluating the efficacy and safety of JDQ443 versus docetaxel in previously treated subjects with locally advanced or metastatic KRAS G12C mutant non-small cell lung cancer |
| 128. A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Patients with Previously Untreated, Locally Advanced, Inoperable or Metastatic Triple-Negative Breast Cancer Whose Tumors do not Express PD-L1 or in Patients Previously Treated With Anti-PD-(L)1 Agents in the Early Setting whose Tumors do Express PD-L1 |
| 129. A Phase 2b Pivotal Study to Evaluate the Efficacy and Safety of Izokibep in Subjects with Moderate to Severe Hidradenitis Suppurativa |
| 130. Part I. Efficacy and Safety of AXL-Inhibitor bemcentinib for the Treatment of Moderate COVID-19 |
| 131. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Dose-finding Study to Assess the Efficacy and Safety of CDX-0159 in Patients with Chronic Spontaneous Urticaria |
| 132. A Randomized, Parallel Design, Multiple-Site Study to Evaluate the noninferiority of generic Clotrimazole 10mg/g cream (Antibiotice SA) compared to Canesten® 10mg/g cream in Patients with Tinea Pedis |
| 133. A Phase II, double-blind, randomized, multiple dose, cross over, threetreatment, three-period, six sequence placebo controlled trial to evaluate efficacy, pharmacokinetics (PK), pharmacodynamics (PD) and safety and tolerability of glycopyrronium (bromide) in children from 6 to less than 12 years of age with asthma |
| 134. A Multicenter, Single-arm, Open-label, Extension, Rollover Study To Evaluate The Long-term Safety And Efficacy Of Ocrelizumab In Patients With Multiple Sclerosis |
| 135. A Phase 1b/3 Study of Bemarituzumab Plus Chemotherapy and Nivolumab Versus Chemotherapy and Nivolumab Alone in Subjects With Previously Untreated Advanced Gastric and Gastroesophageal Junction Cancer With FGFR2b Overexpression (FORTITUDE-102) |
| 136. A phase Ia/Ib, open label, dose-escalation study of the combination of BI 907828 with BI 754091 (ezabenlimab) and BI 754111 and the combination of BI 907828 with BI 754091 (ezabenlimab) followed by expansion cohorts, in patients with advanced solid tumors |
| 137. A single center, open-label, 3-period fixed-sequence, Phase I clinical Study to Evaluate the Effect of BV100 on the Pharmacokinetics of Midazolam and its metabolite 1-hydroxymidazolam in healthy volunteers |
| 138. A Phase 3 Study of the Hepcidin Mimetic Rusfertide (PTG-300) in Patients with Polycythemia Vera |
| 139. A Randomized, Open-label, Phase 3 Trial of Dato-DXd Plus Pembrolizumab vs Pembrolizumab Alone in Treatment-naive Subjects with Advanced or Metastatic PD-L1 High (TPS .50%) Non-small Cell Lung Cancer Without Actionable Genomic Alterations (Tropion-Lung08) |
| 140. A Phase 3 Double-blind, Randomized, Placebo-controlled Study Evaluating the Efficacy and Safety of ELX/TEZ/IVA in Cystic Fibrosis Subjects 6 Years of Age and Older With a Non-F508del ELX/TEZ/IVA-responsive CFTR Mutation |
| 141. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Assess the Efficacy and Safety of KBP-5074, a Mineralocorticoid Receptor Antagonist, in Subjects with Uncontrolled Hypertension Who Have Moderate or Severe (Stage 3b/4) Chronic Kidney Disease |
| 142. A Phase 3, Multicenter Study to Evaluate the Safety and Efficacy of AGN-151586 for the Treatment of Glabellar Lines |
| 143. Study of Females Exposed to Eleclazine |
| 144. A multi-centre, single arm, open-label extension study to evaluate the long-term safety of GSK3511294 (Depemokimab) in adult and adolescent participants with severe asthma with an eosinophilic phenotype from studies 206713 or 213744 |
| 145. A randomised, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of PQ Grass in subjects with seasonal allergic rhinitis and/or rhinoconjunctivitis induced by grass pollen exposure |
| 146. A Phase II/III, Extension Study of Orally Administered PHA-022121 for Acute Treatment of Angioedema Attacks in Patients with Hereditary Angioedema due to C1-Inhibitor Deficiency (Type I or Type II) |
| 147. A long-term extension study to evaluate the long-term safety, tolerability and efficacy of subcutaneous amlitelimab in adult participants with moderate to severe atopic dermatitis who participated in KY1005-CT05 (DRI17366). |
| 148. A Phase 3 Open-Label, Randomized Study of Pirtobrutinib (LOXO-305) versus Ibrutinib in Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (BRUIN-CLL-314) |
| 149. A Phase II/III, prospective, multi-center, randomized, 4-week, doubleblind, placebo-controlled study, designed to determine the safety, tolerability, EEG effects and efficacy of oral doses of 30 mg bid of evenamide (NW-3509) in patients with chronic schizophrenia who are symptomatic on their current second-generation antipsychotic (aripiprazole, clozapine, quetiapine, olanzapine, paliperidone, or risperidone) medication. |
| 150. ASCEND-NASH: A PHASE 2B, RANDOMIZED, MULTI-CENTER, DOUBLEBLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE EFFICACY AND SAFETY OF CRV431 IN ADULT SUBJECTS WITH NONALCOHOLIC STEATOHEPATITIS AND ADVANCED LIVER FIBROSIS |
| 151. A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Pimavanserin for the Treatment of Irritability Associated With Autism Spectrum Disorder |
| 152. A 52-Week Open-Label Extension Study of Pimavanserin in Children and Adolescents with Irritability Associated with Autism Spectrum Disorder (ASD) |
| 153. A randomised, double-blind, placebo-controlled, two-part study to evaluate the pharmacokinetics, safety and tolerability, and preliminary efficacy of two dose levels of golexanolone in subjects with primary biliary cholangitis, fatigue, and cognitive dysfunction. |
| 154. An Open-label Randomized Phase 3 Study of Tucatinib in Combination with Trastuzumab and mFOLFOX6 versus mFOLFOX6 given with or without either Cetuximab or Bevacizumab as First-line Treatment for Subjects with HER2+ Metastatic Colorectal Cancer |
| 155. A 52-week, randomized, double-blind, double-dummy, parallel-group, multi-centre, non-inferiority study to investigate the efficacy and safety of depemokimab compared with mepolizumab in adults with relapsing or refractory Eosinophilic Granulomatosis with Polyangiitis (EGPA) receiving standard of care (SoC) therapy |
| 156. Phase I, Single Blind, Single Escalating Doses Study to evaluate the Safety, Tolerability, Pharmacokinetics and Imaging (CT) Performance of Orally Administered ImageBAT in Healthy Subjects |
| 157. A Multicenter, Open-Label, Dose-Finding, Phase 2 Study Evaluating THIO Sequenced with Cemiplimab (LIBTAYO®) in Subjects with Advanced Non-Small Cell Lung Cancer (NSCLC) |
| 158. A Phase 3, Randomized, Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Guselkumab in Participants with Fistulizing, Perianal Crohn's Disease |
| 159. Efficacy and safety of cagrilintide s.c. 2.4 mg in combination with semaglutide s.c. 2.4 mg (CagriSema s.c. 2.4 mg/2.4 mg) once-weekly in participants withoverweight or obesityand type 2 diabetes |
| 160. A Single Dose, Double-Blind, Parallel Arm, Comparative Pharmacokinetic Study of DRL_AB, US licensed Reference Abatacept (Orencia®) and EU approved Reference (Orencia®), Administered by the Intravenous Route to Male Normal Healthy Volunteers |
| 161. D3-vitaminnal, L-lizinnel és süngomba-kivonattal (Hericium erinaceus) dúsított sajtféleség funkiconális hatásának vizsgálata önkéntes résztvevőkön. |
| 162. ENLIGHTEN 2: A Phase III, Randomized, Blinded, Controlled, Parallel-Group Trial to Evaluate the Efficacy and Safety of LYR-210 for the Treatment of Chronic Rhinosinusitis (CRS) in Adults |
| 163. A randomized, Phase 3, open label study evaluating subcutaneous versus intravenous administration of isatuximab in combination with pomalidomide and dexamethasone in adult patients with relapsed and/or refractory multiple myeloma (RRMM) |
| 164. A randomised, double-blind, parallel, multicentre, multinational study to compare the efficacy, pharmacokinetics, pharmacodynamics, safety and immunogenicity of MB09 (proposed denosumab biosimilar) versus Prolia® (EU-sourced) in postmenopausal women with osteoporosis (SIMBA Study) |
| 165. Investigation of once-weekly semaglutide s.c. dose-response in patients with type 2 diabetes and overweight – a participant- and investigatorblinded and sponsor open-label study |
| 166. CYCLONE 3: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Abemaciclib in Combination with Abiraterone plus Prednisone in Men with High-Risk Metastatic Hormone-Sensitive Prostate Cancer |
| 167. A Phase 3, Multicenter, Randomized, Double blind, Placebo controlled Trial to Evaluate the Efficacy and Safety of Sibeprenlimab Administered Subcutaneously in Subjects with Immunoglobulin A Nephropathy |
| 168. A PHASE 3 TRIAL OF FIANLIMAB (REGN3767, ANTI-LAG-3) + CEMIPLIMAB VERSUS PEMBROLIZUMAB IN PATIENTS WITH PREVIOUSLY UNTREATED UNRESECTABLE LOCALLY ADVANCED OR METASTATIC MELANOMA |
| 169. A Phase 1 study to evaluate safety and tolerability of single and multiple ascending doses of XEN-101 |
| 170. First-in-Human, Double-Blind, Randomised, Vehicle-Controlled Phase I/II Proof of Concept Study to Investigate the Safety, Tolerability, Pharmacokinetics and Efficacy of BEN2293 in Patients with Mild to Moderate Atopic Dermatitis |
| 171. A 52 week study comparing the efficacy and safety of once weekly IcoSema and once weekly semaglutide, both treatment arms with or without oral anti diabetic drugs, in participants with type 2 diabetes inadequately controlled with a GLP 1 receptor agonist. COMBINE 2 |
| 172. A Phase 2, randomised, double-blind, placebo-controlled, dose escalation study to investigate the effects of IRL201805 in participants with moderately to severely active rheumatoid arthritis |
| 173. A Phase 1/2, open-label, multi-center study of the safety and efficacy of KY1044 as single agent and in combination with anti-PD-L1 (atezolizumab) in adult patients with selected advanced malignancies |
| 174. A Phase 2/3 Double-Masked, Randomized, 2-stage, Multicenter Study of the Efficacy and Safety of OCS-01 Eye Drops in Subjects With Diabetic Macular Edema |
| 175. APPRAIS - A 24-week multicentre, randomized, double-blind, placebocontrolled, parallel group trial to evaluate the efficacy, safety and tolerability of orally administered Rabeximod in patients with active, moderate to severe rheumatoid arthritis with inadequate response to methotrexate |
| 176. Clinical study on the safety and efficacy of hydroxychloroquine administered daily as Hydroxychloroquine Meditop 200 mg film-coated tablets for 3 months in 24 patients with rheumatoid arthritis |
| 177. A Phase 2 Open-Label Extension Study to Evaluate the Long-Term Safety and Efficacy of ARO-APOC3 in Adults with Dyslipidemia |
| 178. A multicentre, phase III, double-blind, randomised clinical trial to assess the efficacy and safety of LPRI-CF113 in the treatment of endometriosis versus placebo after 3 medication cycles followed by 3 open-label medication cycles |
| 179. Part I. ALB-TRIAL: Personalized Long-term Human Albumin Treatment in Patients With Decompensated Cirrhosis and Ascites |
| 180. Part II. ALB-TRIAL: Personalized Long-term Human Albumin Treatment in Patients With Decompensated Cirrhosis and Ascites |
| 181. ZEUS - Effects of ziltivekimab versus placebo on cardiovascular outcomes in participants with established atherosclerotic cardiovascular disease, chronic kidney disease and systemic inflammation |
| 182. A Phase III, Open-label, Randomised, Multicentre Study of Ceralasertib Plus Durvalumab Versus Docetaxel in Patients With Advanced or Metastatic Non-Small Cell Lung Cancer Without Actionable Genomic Alterations, and Whose Disease Has Progressed On or After Prior Anti-PD- (L)1 Therapy and Platinum-based Chemotherapy: LATIFY |
| 183. An Open-label Extension Trial to Evaluate the Long-term Safety of KVD900, an Oral Plasma Kallikrein Inhibitor, for On-demand Treatment of Angioedema Attacks in Adolescent and Adult Patients with Hereditary Angioedema Type I or II |
| 184. Part I (Peak) A Phase 3 Randomized, Open-label, Multicenter Clinical Study of CGT9486+Sunitinib vs Sunitinib in Subjects with Locally Advanced, Unresectable, or Metastatic Gastrointestinal Stromal Tumors |
| 185. Part II (Peak) A Phase 3 Randomized, Open-label, Multicenter Clinical Study of CGT9486+Sunitinib vs Sunitinib in Subjects with Locally Advanced, Unresectable, or Metastatic Gastrointestinal Stromal Tumors |
| 186. A Phase 3, Open-Label Study to Evaluate Safety and Efficacy of Epcoritamab in Combination with Rituximab and Lenalidomide (R2) compared to R2 in Subjects with Relapsed or Refractory Follicular Lymphoma (EPCORE™ FL-1) |
| 187. A randomized, double-blind, controlled, multi-center study to evaluate the efficacy and safety of dose de-escalation of orally administered filgotinib in subjects with ulcerative colitis in clinical remission |
| 188. A phase I, open-label, single-center, single-dose, parallel-group study to evaluate the pharmacokinetics of BV100 in participants with mild, moderate, and severe hepatic impairment compared to matched healthy control participants |
| 189. A Phase 3b/4 Randomized, Double-blind, Dose-Flexibility Study of Upadacitinib in Adult Subjects with Moderate to Severe Atopic Dermatitis (Flex-Up) |
| 190. A double-blind, randomized, placebo-controlled multicenter study toinvestigate efficacy and safety of elinzanetant for the treatment of vasomotor symptoms caused by adjuvant endocrine therapy, over 52 weeks in women with, or at high risk for developing hormone-receptor positive breast cancer |
| 191. Endothelial protection in convalecent COVID-19 patients. The effect of Sulodexide on serum levels of biomarkers for endothelial dysfunction. A prospective, randomized, placebo-controlled, investigator-initiated trial. |
| 192. A Randomised, Placebo-Controlled, 3-Arm, Double-Blind, Multicentre, Phase 4 Study to Assess the Efficacy of OM-85 (Broncho Vaxom) Short- and Long-Term Treatment vs. Placebo in the Prevention of Respiratory Tract Infections in Children Aged Between 6 Months and 5 Years with Wheezing Lower Respiratory Illness |
| 193. A Phase III, Randomized, Double-blind, Placebo-controlled, Multicenter Trial to Evaluate the Efficacy and Safety of Diamyd® to Preserve Endogenous Beta Cell Function in Adolescents and Adults with Recently Diagnosed Type 1 Diabetes, Carrying the Genetic HLA DR3-DQ2 Haplotype |
| 194. A trial of prednisolone in combination with SPI-62 or placebo in subjects with polymyalgia rheumatica (PMR) |
| 195. A phase IIIb, multi-center, open-label, randomized study of tolerability and efficacy of oral asciminib versus nilotinib in patients with newly diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase |
| 196. A PROSPECTIVE, RANDOMIZED, OPEN-LABEL PHASE 2 STUDY TO EVALUATE THE SUPERIORITY OF INOTUZUMAB OZOGAMICIN MONOTHERAPY VERSUS ALLR3 FOR INDUCTION TREATMENT OF CHILDHOOD HIGH RISK FIRST RELAPSE B-CELL PRECURSOR ACUTE LYMPHOBLASTIC LEUKAEMIA |
| 197. A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician’s Choice and Pembrolizumab in Patients With Previously Untreated, Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer, Whose Tumors Express PD-L1 |
| 198. Effectiveness and pharmacokinetic /pharmacodynamic study of Furazidin, prolonged- release tablets, 200 mg in the treatment of patients with uncomplicated lower urinary tract infections (acute or recurrent). FUTURE |
| 199. An Open-label, Multicenter Trial to Assess the Safety and Tolerability of Lumateperone as Adjunctive Therapy in the Treatment of Patients with Major Depressive Disorder |
| 200. A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase 3 study to evaluate the safety and efficacy of masitinib as add-on therapy in patients with mild to moderate Alzheimer's disease, treated with standard of care: cholinesterase inhibitors, memantine |
| 201. A multicenter, participant and investigator-blinded, randomized, placebo-controlled Phase 2a study to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of TIN816 in the treatment of patients with sepsis-associated acute kidney injury |
| 202. An Open-label Extension Study to Evaluate Longterm Efficacy and Safety of Odevixibat (A4250) in Children with Biliary Atresia (BOLD-EXT) |
| 203. A Phase 2b, Open-label, Multicenter, Randomized Parallel-Group, Two-Stage, Study of an Immunotherapeutic Treatment DPX-Survivac and Pembrolizumab, with and without Intermittent LowDose Cyclophosphamide, in Subjects with Relapsed/Refractory Diffuse Large BCell Lymphoma (VITALIZE). |
| 204. A randomized, placebo-controlled, double-blind, multi-center, phase III trial to assess the efficacy and safety of trimodulin (BT588) in adult hospitalized subjects with moderate or severe COVID-19 |
| 205. A Phase 2, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Nipocalimab in Participants with Active Idiopathic Inflammatory Myopathies |
| 206. A Multicenter, Randomized, Double-Blind, Parallel-Arm, Phase 3 Study to Compare Efficacy and Safety of BAT2306 with Cosentyx® in Patients with Moderate to Severe Plaque Psoriasis |
| 207. A 24-Week, Multicenter, Randomized, Open-Label, Parallel-Group Trial Comparing the Efficacy and Safety of Insulin Glargine 300 U/mL (Gla-300) and Insulin Degludec 100 U/mL (IDeg-100) in Insulin-Naïve People with Type 2 Diabetes Mellitus and Renal Impairment: TRENT Trial |
| 208. A Phase 3 Open-label Study Evaluating the Long-term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Subjects With Non-F508del CFTR Genotypes |
| 209. Phase 1b, Multicenter, Open-Label Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Efficacy of Mezagitamab (TAK-079) in Patients With Primary IgA Nephropathy in Combination With Stable Background Therapy |
| 210. Phase 1b/2, Open-Label Study to Evaluate Safety and Tolerability of Epcoritamab in Combination with Anti-Neoplastic Agents in Subjects with Non-Hodgkin Lymphoma |
| 211. A Randomized, Multicenter, Open-label, Phase III Study of Lurbinectedin Single-Agent or Lurbinectedin in Combination with Irinotecan versus Investigator’s Choice (Topotecan or Irinotecan) in Relapsed Small Cell Lung Cancer (SCLC) Patients (LAGOON Trial) |
| 212. A Multi-national Phase IIIb, Double-blind, Placebo-controlled Trial to Determine the Safety and Efficacy of STALORAL® Birch 300 IR in Children and Adolescents 5 to 17 Years old with Birch Pollen-induced Allergic Rhinoconjunctivitis with or without Asthma |
| 213. A Phase 3, Randomized, Open-label Study to Evaluate Perioperative Enfortumab Vedotin Plus Pembrolizumab (MK-3475) Versus Neoadjuvant Gemcitabine and Cisplatin in Cisplatin-eligible Participants with Muscle-invasive Bladder Cancer (KEYNOTE-B15 / EV-304) |
| 214. A Phase IIa, randomised, double-blind, placebo-controlled trial to evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of of BI 706321 orally administered for 12 weeks in patients with Crohn`s Disease (CD) receiving ustekinumab induction treatment |
| 215. KontRASt-06: An open-label phase II trial evaluating the activity and safety of JDQ443 single-agent as first-line treatment for patients with locally advanced or metastatic KRAS G12C-mutated non-small cell lung cancer with a PD-L1 expression < 1% or a PD-L1 expression ≥ 1% and an STK11 co-mutation |
| 216. A Randomized Open-Label Phase 3 Study of XL092 + Atezolizumab vs Regorafenib in Subjects with Metastatic Colorectal Cancer |
| 217. PART I (2022-500302-18-00) A Randomized Phase 2 Study of Adjunctive EQU-001 for Uncontrolled Focal Onset Seizures |
| 218. PART I (2022-500290-14-00) A randomized, double-blind, placebo controlled, dose-finding study to assess the efficacy and safety of SAR443122 in adult patients with moderate to severe ulcerative colitis |
| 219. PART II (2022-500290-14-00) A randomized, double-blind, placebo controlled, dose-finding study to assess the efficacy and safety of SAR443122 in adult patients with moderate to severe ulcerative colitis |
| 220. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 2 Induction Study of ADS024 in Participants with Mild to Moderate Ulcerative Colitis (UC) followed by an Optional Open-Label Extension (OLE)" |
| 221. A phase 2, open-label, single-arm, multicenter study of SOT101 in combination with pembrolizumab to evaluate the efficacy and safety in patients with selected advanced/refractory solid tumors |
| 222. A Multicenter, Randomized, Double-Blind, Parallel-Controlled Phase III Clinical Study to Evaluate the Efficacy and Safety of Pertuzumab Biosimilar HLX11 vs. EU-Perjeta® in the Neoadjuvant Therapy of HER2-Positive and HR-Negative Early-stage or Locally Advanced Breast Cancer |
| 223. A pivotal Phase II randomised, multi-centre, open label study to evaluate the efficacy and safety of MB CART2019.1 compared to standard of care therapy in participants with relapsed/refractory diffuse large B cell lymphoma (R R DLBCL), who are not eligible for high dose chemotherapy and autologous stem cell transplantation |
| 224. A Phase 1/2a, First-in-human Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of HDP-101 in Patients with Plasma Cell Disorders Including Multiple Myeloma |
| 225. A Phase 2, Randomized, Double-blind, Placebo-controlled Trial to Evaluate the Efficacy, Safety, and Tolerability of Two Fixed Doses (15 mg and 30 mg QD) of CVL-231 in Participants With Schizophrenia Experiencing an Acute Exacerbation of Psychosis |
| 226. A Phase 1, open-label, study to investigate the pharmacokinetics and safety of remibrutinib (LOU064) in participants with hepatic impairment compared to matched healthy participants with normal hepatic function |
| 227. Efficacy and safety of SAR441344 in the treatment of Systemic Lupus Erythematosus: A randomized, double blind, placebo-controlled, Phase 2, proof of concept study |
| 228. A Multicenter, Randomized, Double-Blind, Parallel Group, Placebo-Controlled Trial to Evaluate the Effect of In-Hospital Initiation of Dapagliflozin on Clinical Outcomes in Patients Who Have Been Stabilized During Hospitalization for Acute Heart Failure |
| 229. A Phase 1 Single Center Open-label, Non-randomized, Fixed Sequence Study in Healthy Volunteers to Assess the Pharmacokinetics (PK) of BV100 When Administered Alone and With Itraconazole |
| 230. PART I A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Oral Brepocitinib in Adults with Dermatomyositis |
| 231. PART II A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Oral Brepocitinib in Adults with Dermatomyositis |
| 232. A PHASE 2B RANDOMIZED, DOUBLE-BLIND, ACTIVE-AND PLACEBO-CONTROLLED, PARALLEL-GROUP, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF INDUCTION AND MAINTENANCE COMBINATION THERAPY WITH GUSELKUMAB AND GOLIMUMAB IN PARTICIPANTS WITH MODERATELY TO SEVERELYACTIVE CROHN’S DISEASE – DUET-CD |
| 233. A 52-week, Phase 2, Open-label Trial to Evaluate the Long-term Safety and Tolerability of CVL-231 in Adult Participants With Schizophrenia |
| 234. A multi-center, randomized, double-blind, parallel-group, 20-week dose-finding study to evaluate efficacy, safety, and tolerability of XXB750 in patients with resistant hypertension |
| 235. An open-label, non-randomized Phase I investigation of human ADME (absorption, distribution, metabolism and excretion) and absolute oral bioavailability of BI 907828 in patients with advanced solid tumours |
| 236. A Phase III, Randomised, Open-label, Multicentre, Global Study of Datopotamab Deruxtecan (Dato-DXd) in Combination With Durvalumab and Carboplatin Versus Pembrolizumab in Combination With Platinum-based Chemotherapy for the First-line Treatment of Patients With Locally Advanced or Metastatic NSCLC Without Actionable Genomic Alterations (D926NC00001; AVANZAR |
| 237. Prospective, randomized, double-blind, placebo-controlled, multicenter study to investigate the efficacy and safety of NT 201 in the treatment of lower limb spasticity caused by stroke or traumatic brain injury in adult subjects, followed by an open label extension with or without combined upper limb treatment |
| 238. A PHASE 1, RANDOMIZED, DOUBLE-BLIND, SPONSOR-OPEN, PLACEBO-CONTROLLED, FIRST-IN-HUMAN TRIAL TO EVALUATE THE SAFETY, TOLERABILITY, AND PHARMACOKINETICS OF PF-06755347 AFTER SINGLE ASCENDING INTRAVENOUS AND SUBCUTANEOUS DOSING IN HEALTHY ADULT MALE PARTICIPANTS AND OPEN-LABEL AFTER SINGLE SUBCUTANEOUS DOSING IN MALE AND FEMALE PARTICIPANTS WITH PERSISTENT OR CHRONIC PRIMARY IMMUNE THROMBOCYTOPENIA |
| 239. PART I A Phase 1-2, Open-Label Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Activity of Tolinapant in Combination with Oral Decitabine/Cedazuridine and Oral Decitabine/Cedazuridine Alone in Subjects with Relapsed/Refractory Peripheral T-cell Lymphoma |
| 240. PART II A Phase 1-2, Open-Label Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Activity of Tolinapant in Combination with Oral Decitabine/Cedazuridine and Oral Decitabine/Cedazuridine Alone in Subjects with Relapsed/Refractory Peripheral T-cell Lymphoma |
| 241. AN INTERVENTIONAL EFFICACY AND SAFETY, PHASE 2, RANDOMIZED, DOUBLE-BLIND, 3-ARM STUDY TO INVESTIGATE NIRMATRELVIR/RITONAVIR IN NONHOSPITALIZED PARTICIPANTS AT LEAST 12 YEARS OF AGE WITH SYMPTOMATIC COVID-19 WHO ARE IMMUNOCOMPROMISED |
| 242. A PHASE 2B RANDOMIZED, DOUBLE-BLIND, ACTIVE-AND PLACEBO-CONTROLLED, PARALLEL-GROUP, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF INDUCTION AND MAINTENANCE COMBINATION THERAPY WITH GUSELKUMAB AND GOLIMUMAB IN PARTICIPANTS WITH MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS DUET-UC |
| 243. A Single Group Treatment, Phase 2 study to investigate Pharmacokinetics, Safety and Tolerability of Cefepime-Enmetazobactam administered by intra-venous infusion over 2 hours in Male or Female Participants from birth to less than 18 years of age hospitalized with complicated urinary tract infections (cUTI) including Acute Pyelonephritis (AP) |
| 244. A randomized, double-blind, placebo-controlled, parallel-group, dose ranging study to assess the efficacy, safety, and tolerability of subcutaneous amlitelimab in adult participants with moderate to-severe asthma |
| 245. PART I A Phase 3, Multicenter, Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of Contezolid Acefosamil and Contezolid Compared to Linezolid Administered Intravenously and Orally to Adults with Moderate or Severe Diabetic Foot Infections |
| 246. PART II A Phase 3, Multicenter, Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of Contezolid Acefosamil and Contezolid Compared to Linezolid Administered Intravenously and Orally to Adults with Moderate or Severe Diabetic Foot Infections |
| 247. A 6-month prospective, randomized, double-blind, placebo-controlled clinical trial investigating the efficacy, safety, and tolerability of two different doses of buntanetap or placebo in patients with early Parkinson’s disease |
| 248. PART I A Phase 3, Two-stage, Randomized, Multicenter, Open-label Study Comparing CC-92480 (BMS-986348), Carfilzomib, and Dexamethasone (480Kd) Versus Carfilzomib and Dexamethasone (Kd) in Participants with Relapsed or Refractory Multiple Myeloma (RRMM) |
| 249. PART II A Phase 3, Two-stage, Randomized, Multicenter, Open-label Study Comparing CC-92480 (BMS-986348), Carfilzomib, and Dexamethasone (480Kd) Versus Carfilzomib and Dexamethasone (Kd) in Participants with Relapsed or Refractory Multiple Myeloma (RRMM) |
| 250. Part I. A double-blind, randomized, placebo-controlled, interventional, multicenter, phase III clinical trial to investigate the safety and efficacy of ABCB5-positive mesenchymal stromal cells (ABCB5+ MSCs) on epidermolysis bullosa (EB) |
| 251. Part II. A double-blind, randomized, placebo-controlled, interventional, multicenter, phase III clinical trial to investigate the safety and efficacy of ABCB5-positive mesenchymal stromal cells (ABCB5+ MSCs) on epidermolysis bullosa (EB) |
| 252. Part I. A Phase 2 Multi-Cohort, Open-Label, Multi-Center Clinical Study Evaluating the Efficacy and Safety of Disitamab Vedotin (RC48-ADC) Alone and in Combination with Pembrolizumab in Subjects with Locally-Advanced Unresectable or Metastatic Urothelial Carcinoma That Expresses HER2 |
| 253. Part II. A Phase 2 Multi-Cohort, Open-Label, Multi-Center Clinical Study Evaluating the Efficacy and Safety of Disitamab Vedotin (RC48-ADC) Alone and in Combination with Pembrolizumab in Subjects with Locally-Advanced Unresectable or Metastatic Urothelial Carcinoma That Expresses HER2 |
| 254. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of LY3540378 in Adults with Worsening Chronic Heart Failure with Preserved Ejection Fraction (HFpEF) |
| 255. A Phase 3b Extension Study to Evaluate the Long-term Safety of Vedolizumab Intravenous in Pediatric Patients With Ulcerative Colitis or Crohn's Disease |
| 256. A multicenter, randomized, double-blind, placebocontrolled, parallel-arm study to assess the efficacy, safety, and tolerability of AVP-786 (deudextromethorphan hydrobromide [d6-DM]/quinidine sulfate [Q]) for the treatment of negative symptoms of schizophrenia |
| 257. A Phase 2/3, Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group, 2-Arm, Multicenter, Operationally Seamless Study to Evaluate the Efficacy, Safety, Tolerability, Pharmacodynamics, "Pharmacokinetics, and Immunogenicity of Efgartigimod PH20 SC in Participants Aged 18 Years and Older With Active Idiopathic Inflammatory Myopathy |
| 258. A Phase 3b/4 Randomized, Open-label, Efficacy Assessor Blinded Study, Comparing the Safety and Assessor Blinded Efficacy of Upadacitinib to Dupilumab in Subjects with Moderate to Severe Atopic Dermatitis (Level-Up) |
| 259. Phase 3, randomised, double-blind, multi-centre trial to evaluate the efficacy, safety, and tolerability of brodalumab treatment compared to placebo (blinded) and ustekinumab (open-label) in adolescent subjects (12-17 years of age) with moderate-to-severe plaque psoriasis Short Title: Efficacy and safety of brodalumab in adolescents from 12 to 17 years of age with moderate-to-severe plaque psoriasis |
| 260. A Randomized, Open-Label, Multicenter Phase 3 Trial of Domvanalimab, Zimberelimab, and Chemotherapy Versus Nivolumab and Chemotherapy in Participants with Previously Untreated Locally Advanced Unresectable or Metastatic Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma |
| 261. An Open-Label Study of CK-3773274 for Patients with Symptomatic Hypertrophic Cardiomyopathy (HCM) |
| 262. Part I. A PHASE 2A MULTICENTER, OBSERVER-BLINDED, RANDOMIZED 2 ARM STUDY TO INVESTIGATE PHARMACOKINETICS, SAFETY, TOLERABILITY AND EFFICACY OF INTRAVENOUS AZTREONAM-AVIBACTAM ± METRONIDAZOLE COMPARED TO BEST AVAILABLE THERAPY (BAT) IN PEDIATRIC PARTICIPANTS 9 MONTHS TO LESS THAN 18 YEARS OF AGE WITH SERIOUS GRAM-NEGATIVE BACTERIAL INFECTIONS INCLUDING COMPLICATED INTRA-ABDOMINAL INFECTION |
| 263. Part II. A PHASE 2A MULTICENTER, OBSERVER-BLINDED, RANDOMIZED 2 ARM STUDY TO INVESTIGATE PHARMACOKINETICS, SAFETY, TOLERABILITY AND EFFICACY OF INTRAVENOUS AZTREONAM-AVIBACTAM ± METRONIDAZOLE COMPARED TO BEST AVAILABLE THERAPY (BAT) IN PEDIATRIC PARTICIPANTS 9 MONTHS TO LESS THAN 18 YEARS OF AGE WITH SERIOUS GRAM-NEGATIVE BACTERIAL INFECTIONS INCLUDING COMPLICATED INTRA-ABDOMINAL INFECTION |
| 264. A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study of the Efficacy, Safety, and Pharmacokinetics of Two Doses of INV-202 in Patients with Diabetic Kidney Disease |
| 265. An Open-Label, Safety, Tolerability, and Proof-of-Concept Study of Oral BCX9930 Therapy in Subjects with Complement 3 Glomerulopathy, Immunoglobulin A Nephropathy, or Primary Membranous Nephropathy |
| 266. Part I Phase 3, single-arm, multicenter, multinational, open-label, one-way crossover study to investigate the efficacy and safety of fitusiran prophylaxis in male participants aged ≥ 12 years with severe hemophilia A or B, with or without inhibitory antibodies to factor VIII or IX |
| 267. Part II Phase 3, single-arm, multicenter, multinational, open-label, one-way crossover study to investigate the efficacy and safety of fitusiran prophylaxis in male participants aged ≥ 12 years with severe hemophilia A or B, with or without inhibitory antibodies to factor VIII or IX |
| 268. A multicenter study of secukinumab, with a randomized double-blind, placebo-controlled withdrawal-retreatment period, to evaluate maintenance of response in participants with non-radiographic axial spondyloarthritis who achieved remission |
| 269. A Phase 2, Randomized, Open-label, Platform Study Utilizing a Master Protocol to Evaluate Novel Immunotherapy Combinations in Participants with Previously Untreated, Locally Advanced/Metastatic, Programmed Death Ligand 1-Selected Non-Small-Cell Lung Cancer |
| 270. An Open-label Extension (OLE) Phase 3 Trial to Assess the Safety of Intravitreal Administration of avacincaptad pegol (Complement C5 Inhibitor) in Patients with Geographic Atrophy Who Previously Completed Phase 3 Study ISEE2008 (GATHER2) |
| 271. A Double-blind, Randomized, Placebo- and Active-Comparator Controlled Study to Evaluate the Efficacy of Inclisiran as Monotherapy in Patients with Primary Hypercholesterolemia Not Receiving Lipid-Lowering Therapy (VictORION-Mono) |
| 272. A 14-Week Phase 2b, RandomizEd, Double-BLind, Dose-Ranging Study to Determine the PharmacokInetics, Efficacy, Safety, and Tolerability of TEV-48574 in Adult PatiEnts with Moderate to Severe Ulcerative Colitis or Crohn’s Disease (RELIEVE UCCD) |
| 273. A Phase 2/3, Multicenter, Randomized, Double-blind Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Oral Ozanimod (RPC1063) in Pediatric Participants with Moderately to Severely Active Crohn’s Disease with an Inadequate Response to Conventional Therapy |
| 274. Phase I, Randomised, Blinded, Placebo-controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AZD8630 in Healthy Adult Subjects (Part A) and Adults with Asthma on Medium to High Dose Inhaled Corticosteroids and Long-acting Beta-agonists (Part B) |
| 275. A Multicenter, Randomized, Dose-Blind, Phase 3 Long-Term Extension Study to Evaluate Continuous Safety and Efficacy of BIIB059 in Adult Participants with Active Systemic Lupus Erythematosus |
| 276. A Randomized, Double-Blind, International Multicenter, Parallel-Controlled Phase III Clinical Study to Evaluate Recombinant Anti-RANKL Human Monoclonal Antibody Injection (HLX14) Versus Denosumab Injection (Prolia®) in Postmenopausal Women with Osteoporosis at High Risk of Fracture |
| 277. Part I. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, 2-Stage Trial to Evaluate Efficacy and Safety of Telitacicept Compared to Placebo in Patients with Moderately to Severely Active Systemic Lupus Erythematosus |
| 278. Part II. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, 2-Stage Trial to Evaluate Efficacy and Safety of Telitacicept Compared to Placebo in Patients with Moderately to Severely Active Systemic Lupus Erythematosus |
| 279. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of GS-5245 for the Treatment of COVID-19 in Participants With High-Risk for Disease Progression |
| 280. A Phase 3b Randomized, Double-Blind, Multi-Center Study to Compare the Safety and Efficacy of Omadacycline IV/PO to Moxifloxacin IV/PO for Treating Adult Subjects with Community-Acquired Bacterial Pneumonia (CABP) |
| 281. Part I. Efficacy and safety of Baricitinib for the treatment of severe COVID-19 A double bind, multicentre, randomized, placebo-controlled, phase 3 trial to investigate the safety and efficacy of baricitinib + standard of care (SoC) compared with placebo + SoC on the occurrence of death in male and female participants aged > 18 years with severe COVID-19 |
| 282. Part II. Efficacy and safety of Baricitinib for the treatment of severe COVID-19 A double bind, multicentre, randomized, placebo-controlled, phase 3 trial to investigate the safety and efficacy of baricitinib + standard of care (SoC) compared with placebo + SoC on the occurrence of death in male and female participants aged > 18 years with severe COVID-19 |
| 283. A Phase 2, Multicenter, Randomized, Double-blind, Placebo controlled Study to Assess the Safety, Pharmacokinetics, and Efficacy of KPL-404 in Subjects with Moderate to Severe, Active Rheumatoid Arthritis with Inadequate Response or Intolerance to at Least One Biologic Disease-modifying Anti rheumatic Drug or a Janus Kinase Inhibitor |
| 284. Part I. A Parallel-group (2-Arm), Randomized, Double blind, 12-week Trial to Evaluate the Efficacy and Safety of MC2-25 Cream and MC2-25 Vehicle in Subjects with Chronic Kidney Disease-associated Pruritus (CKD-aP) |
| 285. Part II. A Parallel-group (2-Arm), Randomized, Double blind, 12-week Trial to Evaluate the Efficacy and Safety of MC2-25 Cream and MC2-25 Vehicle in Subjects with Chronic Kidney Disease-associated Pruritus (CKD-aP) |
| 286. Part I. A Phase 3, Randomized Study to Compare the Efficacy and Safety of Nemtabrutinib Versus Chemoimmunotherapy for Previously Untreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Without TP53 Aberrations (BELLWAVE-008) |
| 287. Part II. A Phase 3, Randomized Study to Compare the Efficacy and Safety of Nemtabrutinib Versus Chemoimmunotherapy for Previously Untreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Without TP53 Aberrations (BELLWAVE-008) |
| 288. A double blind, randomized, placebo-controlled trial evaluating the efficacy and safety of BI 1015550 over at least 52 weeks in patients with Idiopathic Pulmonary Fibrosis (IPF) |
| 289. A Phase II Multicenter, open label, non-randomized study of neoadjuvant and Adjuvant Treatment with IPH5201 and durvalumab in patients with resectable, early-Stage (II to IIIA) Non-Small Cell Lung Cancer (MATISSE) |
| 290. A Phase 3, Randomized, Open-Label Study to Evaluate Safety and Efficacy of Epcoritamab in Combination with R-CHOP compared to R-CHOP in Subjects with Newly Diagnosed Diffuse Large B-Cell Lymphoma (DLBCL) |
| 291. A double blind, randomized, placebo-controlled trial evaluating the efficacy and safety of BI 1015550 over at least 52 weeks in patients with Progressive Fibrosing Interstitial Lung Diseases (PF-ILDs) |
| 292. A 2-Part Seamless Part A (Phase 2)/Part B (Phase 3) Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of BIIB059 in Participants with Active Subacute Cutaneous Lupus Erythematosus and/or Chronic Cutaneous Lupus Erythematosus with or without Systemic Manifestations and Refractory and/or Intolerant to Antimalarial Therapy (AMETHYST) |
| 293. Adjustable brodalumab dosage regimen compared with standard brodalumab treatment for 52 weeks in subjects with moderate-to-severe plaque psoriasis and ł120 kg body weight; ADJUST |
| 294. A Randomized, Double-blind, Multicenter, Parallel-Group, Placebo-controlled Study to Evaluate the Efficacy, Safety, and Tolerability of Aticaprant 10 mg as Adjunctive Therapy in Adult Participants with Major Depressive Disorder (MDD) with Moderate-to-Severe Anhedonia and Inadequate Response to Current Antidepressant Therapy |
| 295. A Randomized, Double-blind, Placebo-Controlled, Multi-Center Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamic of Single Ascending Doses of REGN5381, an NPR1 Agonist, in Heart Failure Patients with Elevated Pulmonary Capillary Wedge Pressure and Reduced Left Ventricular Ejection Fraction |
| 296. NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease; a prospective randomized trial |
| 297. Phase 3 Randomized, Controlled Study of Blinatumomab Alternating With Low-intensity Chemotherapy Versus Standard of Care for Older Adults With Newly Diagnosed Philadelphia-negative B-cell Precursor Acute Lymphoblastic Leukemia With Safety Run-in |
| 298. A Phase 2a, Multicenter, Randomized, Double-blind Study Evaluating the Efficacy and Safety of Subcutaneously Administered Guselkumab and Golimumab Combination Therapy in Participants with Active Psoriatic Arthritis – AFFINITY |
| 299. A phase III, randomized, double-blind study of ianalumab (VAY736) versus placebo in addition to first-line corticosteroids in primary immune thrombocytopenia (VAYHIT1) |
| 300. A multicenter, international, randomized, active comparator-controlled, double-blind, double-dummy, parallel-group, 2-arm, Phase 3 study to compare the efficacy and safety of the oral FXIa inhibitor asundexian (BAY 2433334) with apixaban for the prevention of stroke or systemic embolism in male and female participants aged 18 years and older with atrial fibrillation at risk for stroke. |
| 301. A phase 2, randomized, double-blind, placebo controlled, dose ranging, dose finding, parallel group study to assess efficacy and safety of pf-07081532, and open label oral semaglutide, in adults with type 2 diabetes mellitus (t2dm) inadequately controlled on metformin, and separately pf-07081532 compared to matching placebo in adults with obesity but without t2dm |
| 302. A multicenter, international, randomized, placebo controlled, double-blind, parallel group and event driven Phase 3 study of the oral FXIa inhibitor asundexian (BAY 2433334) for the prevention of ischemic stroke in male and female participants aged 18 years and older after an acute non-cardioembolic ischemic stroke or high-risk TIA |
| 303. Phase 2, Randomized, Parallel-Group, Double-Blind, Placebo-Controlled Study of Sonelokimab in Patients with Active Psoriatic Arthritis |
| 304. A randomized, double-blind, parallel group, placebo-controlled multicenter phase 3 study to evaluate efficacy, safety and tolerability of two regimens of ianalumab on top of standard-of-care therapy in patients with systemic lupus erythematosus (SIRIUS-SLE 1) |
| 305. A Randomized, Double-Blind, International Multicenter, Phase HI Study to Evaluate the Anti—Tumor Efficacy and Safety of I-ILXIO (Recombinant Humanized Anti—PD-I Monoclonal Antibody Injection) or Placebo in Combination with Chemotherapy (Carboplatin/Cisplatin-Etoposide) and Concurrent Radiotherapy in Patients with Limited—Stage Small Cell Lung Cancer (LS-SCLC) |
| 306. A phase 3 randomized, double-blind study of ianalumab (VAY736) versus placebo in addition to eltrombopag in patients with primary immune thrombocytopenia (ITP) who had insufficient response or relapsed after first line steroid treatment (VAYHIT2) |
| 307. A randomized, double-blind, placebo-controlled, parallelgroup, 12-week Proof-of-Concept (PoC) study to assess the efficacy, safety, and tolerability of rilzabrutinib in participants with moderate-to-severe asthma who are not well controlled on inhaled corticosteroid (ICS) plus longacting a 2 adrenergic agonist (LABA) therapy |
| 308. Effect and safety of semaglutide 7.2 mg once-weekly in participants with obesity |
| 309. Effect and safety of semaglutide 7.2 mg once-weekly in participants with obesity and type 2 diabetes |
| 310. A randomized, open-label, ravulizumab-controlled, noninferiority study to evaluate the efficacy and safety of pozelimab and cemdisiran combination therapy in patients with paroxysmal nocturnal hemoglobinuria who are complement inhibitor treatment-naive or have not recently received complement inhibitor therapy |
| 311. A Randomized, Open-Label Eculizumab and Ravulizumab Controlled, Non-Inferiority Study to Evaluate the Efficacy and Safety of Pozelimab and Cemdisiran Combination Therapy in Patients with Paroxysmal Nocturnal Hemoglobinuria Who are Currently Treated with Eculizumab or Ravulizumab |
| 312. A Randomized, Double-blind, Placebo-controlled Clinical Study to Evaluate Mavacamten in Adults with Symptomatic Nonobstructive Hypertrophic Cardiomyopathy |
| 313. A phase 3, randomized, double-blind, study to assess efficacy and safety of ianalumab (VAY736) versus placebo in warm autoimmune hemolytic anemia (wAIHA) patients who failed at least one line of treatment (VAYHIA) |
| 314. LOGGIC/FIREFLY-2: A Phase 3, Randomized, International Multicenter Trial of DAY101 Monotherapy Versus Standard of Care Chemotherapy in Patients with Pediatric Low-Grade Glioma Harboring an Activating RAF Alteration Requiring First-Line Systemic Therapy |
| 315. Part I. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Oral Once-Daily LY3473329 in Adults with Elevated Lipoprotein(a) at High Risk for Cardiovascular Events |
| 316. Part II. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Oral Once-Daily LY3473329 in Adults with Elevated Lipoprotein(a) at High Risk for Cardiovascular Events |
| 317. A Phase I, Double-blind, Randomized, Three-arm, Parallel Group, Study to Compare the Pharmacokinetics and Safety of a Single Subcutaneous Dose of PBP1502, EU-Humira®,and US-Humira®in Healthy Male and Female Subjects |
| 318. A Randomized, Controlled, Open-label, Multicenter, Inferentially Seamless Phase 2/3 Study of Ibrutinib in Combination With Rituximab Versus Physician’s Choice of Lenalidomide Plus Rituximab or Bortezomib Plus Rituximab in Participants with Relapsed or Refractory Mantle Cell Lymphoma |
| 319. A Phase II, Open-label, Multicentre, Randomised Study of Neoadjuvant and Adjuvant Treatment in Patients with Resectable, Early-stage (II to IIIB) Non-small Cell Lung Cancer (NeoCOAST-2) |
| 320. An open-label, single arm, multicenter extension study to evaluate long-term safety and tolerability of inclisiran in participants with heterozygous or homozygous familial hypercholesterolemia who have completed the adolescent ORION-16 or ORION-13 studies (VICTORION-PEDS-OLE) |
| 321. A Phase 2 Randomized, Placebo-controlled, Double-blind, Dose-ranging Study to Evaluate the Efficacy, Safety and Tolerability of AMG 133 in Adult Subjects With Overweight or Obesity, With or Without Type 2 Diabetes Mellitus |
| 322. A randomized, multi-centric, placebo-controlled, participant and investigator-blinded study to evaluate the safety, tolerability and efficacy of TIN816 in adult patients at risk for acute kidney injury following cardiac surgery |
| 323. Part I. A Phase 3, Randomized, Double-blind, Placebo-Controlled Study to Investigate the Effect of Tirzepatide on the Reduction of Morbidity and Mortality in Adults with Obesity |
| 324. Part II. A Phase 3, Randomized, Double-blind, Placebo-Controlled Study to Investigate the Effect of Tirzepatide on the Reduction of Morbidity and Mortality in Adults with Obesity |
| 325. Part II. An Open-label Extension Study to Evaluate Longterm Efficacy and Safety of Odevixibat (A4250) in Children with Biliary Atresia (BOLD-EXT) |
| 326. An Open-label, Long-term, Safety and Efficacy Study of Aticaprant as Adjunctive Therapy in Adult and Elderly Participants With Major Depressive Disorder (MDD) |
| 327. A PHASE 2, DOUBLE-BLIND, RANDOMIZED, PLACEBO-CONTROLLED, 4-ARM STUDY TO INVESTIGATE SYMPTOMS, FUNCTION, HEALTH-RELATED QUALITY OF LIFE AND SAFETY WITH REPEATED SUBCUTANEOUS ADMINISTRATION OF PONSEGROMAB VERSUS PLACEBO IN ADULT PARTICIPANTS WITH HEART FAILURE |
| 328. Part I. ALB-TRIAL: Personalized Long-term Human Albumin Treatment in Patients With Decompensated Cirrhosis and Ascites |
| 329. Part II. ALB-TRIAL: Personalized Long-term Human Albumin Treatment in Patients With Decompensated Cirrhosis and Ascites |
| 330. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of the Efficacy and Safety of Parsaclisib in Participants with Primary Warm Autoimmune Hemolytic Anemia (PATHWAY) |
| 331. An Open-Label, Multicenter, Phase 1/2 Trial of GEN3014 (HexaBody®-CD38) in Relapsed or Refractory Multiple Myeloma and Other Hematologic Malignancies |
| 332. A phase I, open-label, single-center, adaptive, multiple-dose trial to evaluate the safety, tolerability and pharmacokinetics of norucholic acid in participants with mild, moderate, and severe hepatic impairment compared to matched healthy control participants |
| 333. A Multicenter, Randomized, Double-blind, Placebo-controlled 12-Week Study to Evaluate the Safety and Efficacy of Oral Difelikefalin in Advanced Chronic Kidney Disease Subjects with Moderate-to-Severe Pruritus and not on Dialysis with an up to 52-Week Long-term Extension |
| 334. A Phase 3, Open-label Study Evaluating the Long-term Safety and Efficacy of VX-121 Combination Therapy in Subjects With Cystic Fibrosis |
| 335. A multicenter, double-blind, placebo-controlled, randomized withdrawal and open-label extension study followed by long-term open-label treatment cycles to assess the efficacy, safety and tolerability of remibrutinib (LOU064) in adult chronic spontaneous urticaria patients who completed the preceding remibrutinib Phase 3 studies |
| 336. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of ABBV-154 in Subjects with Moderately to Severely Active Crohn's Disease (CD): AIM-CD |
| 337. Part I. A Phase 2/3, Multicenter, Randomized, Double-blind, Placebocontrolled Trial of the Safety and Efficacy of Flexible Doses of SEP-363856 as Adjunctive Therapy in the Treatment of Adults with Major Depressive Disorder |
| 338. Part II. A Phase 2/3, Multicenter, Randomized, Double-blind, Placebocontrolled Trial of the Safety and Efficacy of Flexible Doses of SEP-363856 as Adjunctive Therapy in the Treatment of Adults with Major Depressive Disorder |
| 339. A Phase 2 Study to Evaluate the Efficacy and Safety of MK-1026 in Participants with Hematologic Malignancies |
| 340. A Multicentre, Open label, Randomised, Controlled, Basket, Pragmatic, Phase II, Clinical and Translational Study to Determine the Efficacy and Safety of Plitidepsin versus Control in Immunocompromised Adult Patients with Symptomatic COVID-19 requiring Hospital Care (NEREIDA) |
| 341. Safety and efficacy of inhaled pegylated adrenomedullin (PEG-ADM) in patients suffering from Acute Respiratory Distress Syndrome (ARDS): a double-blind, randomized, placebo-controlled, multicenter Phase 2a/b clinical trial |
| 342. Prospective, open-label, single-arm, multicentre Phase 3 study to evaluate the pharmacokinetics, efficacy, tolerability, and safety of subcutaneous human immunoglobulin (Newnorm) in patients with primary immunodeficiency diseases |
| 343. A phase 1b double-blind, randomized, placebo-controlled, multicenter, dose titration study to evaluate the safety, tolerability, and pharmacokinetics of 4 weeks treatment with BAY 2413555 in participants with heart failure and implanted cardiac defibrillator or cardiac resynchronization devices |
| 344. A phase I/II, open-label, multi-cohort study to evaluate the efficacy and safety of cevostamab in prior b cell maturation antigen-exposed patients with relapsed/refractory multiple myeloma |
| 345. A Phase 3 Randomized Study Comparing Bortezomib, Lenalidomide and Dexamethasone (VRd) followed by Ciltacabtagene Autoleucel, a Chimeric Antigen Receptor T cell (CAR-T) Therapy Directed Against BCMA versus Bortezomib, Lenalidomide, and Dexamethasone (VRd) followed by Lenalidomide and Dexamethasone (Rd) Therapy in Participants with Newly Diagnosed Multiple Myeloma for Whom Hematopoietic Stem Cell Transplant is Not Planned as Initial Therapy |
| 346. An Open-label Study to Evaluate the Long-term Safety of BCX9930 Monotherapy in Subjects with Paroxysmal Nocturnal Hemoglobinuria Who Previously Received BCX9930 in a BioCryst sponsored Study |
| 347. An open label, Phase IV study to assess immunological changes in patients with glioblastoma multiforme treated with bevacizumab infusion |
| 348. A Phase 1 Open-label Study to Evaluate the Pharmacokinetics and Pharmacodynamics of a Single Oral Dose of BCX10013 in Subjects with Varying Degrees of Renal Impairment |
| 349. Part I. A Phase 2b, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of 3 Active Dose Regimens of MORF-057 in Adults with Moderately to Severely Active Ulcerative Colitis (EMERALD-2) |
| 350. Part II. A Phase 2b, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of 3 Active Dose Regimens of MORF-057 in Adults with Moderately to Severely Active Ulcerative Colitis (EMERALD-2) |
| 351. Part I. A Phase 2a/b, Randomized, Double-blind, Placebo-controlled, Parallel Group, Multicenter, Clinical Study to Evaluate the Efficacy and Safety of OG-6219 in 3 Dose Levels, in Women 18 to 49 Years of Age with Moderate to Severe Endometriosis-related Pain |
| 352. Part I. A Phase 2b, Double-Blind, Placebo-Controlled Study to Evaluate Peresolimab in Adult Participants with Moderately-to-Severely Active Rheumatoid Arthritis |
| 353. Part II. A Phase 2b, Double-Blind, Placebo-Controlled Study to Evaluate Peresolimab in Adult Participants with Moderately-to-Severely Active Rheumatoid Arthritis |
| 354. Part I. A Phase 2/3 Randomized, Placebo-Controlled, Double-blind, Clinical Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Vericiguat in Pediatric Participants with Heart Failure due to Left Ventricular Systolic Dysfunction (VALOR) |
| 355. Part II. A Phase 2/3 Randomized, Placebo-Controlled, Double-blind, Clinical Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Vericiguat in Pediatric Participants with Heart Failure due to Left Ventricular Systolic Dysfunction (VALOR) |
| 356. Part I. A randomized, placebo-controlled, double-blind, multi-center, phase III trial to assess the efficacy and safety of trimodulin (BT588) in adult hospitalized subjects with severe community-acquired pneumonia (sCAP) |
| 357. A Phase 3, 52-Week, Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Efficacy and Safety Study with Open-Label Extension of BLU-5937 in Adult Participants with Refractory Chronic Cough, Including Unexplained Chronic Cough (CALM-1) |
| 358. ENCORAFENIB/BINIMETINIB MASTER PROTOCOL: AN OPEN-LABEL CONTINUATION STUDY FOR PARTICIPANTS CONTINUING FROM ENCORAFENIB/BINIMETINIB CLINICAL STUDIES |
| 359. Part I. A Randomized, Open-Label, Phase 3 Study of Adjuvant Imlunestrant vs Standard Adjuvant Endocrine Therapy in Patients who have Previously Received 2 to 5 years of Adjuvant Endocrine Therapy for ER+, HER2- Early Breast Cancer with an Increased Risk of Recurrence |
| 360. Part II. A Randomized, Open-Label, Phase 3 Study of Adjuvant Imlunestrant vs Standard Adjuvant Endocrine Therapy in Patients who have Previously Received 2 to 5 years of Adjuvant Endocrine Therapy for ER+, HER2- Early Breast Cancer with an Increased Risk of Recurrence |
Módosítási kérelmek
| Hónap | Kérelmek száma |
| Január | 189 |
| Február | 148 |
| Március | 268 |
| Április | 124 |
| Május | 220 |
| Június | 170 |
| Július | 216 |
| Augusztus | 179 |
| Szeptember | 200 |
| Október | 164 |
| November | 180 |
| December | 166 |
Az ETT KFEB által tárgyalt klinikai vizsgálati kérelmek (2023)
| Sorszám és cím |
|---|
| 1. A Phase 3, Multi-center, Randomized, Quadruplemasked, Placebo-controlled Study of Batoclimab for the Treatment of Participants with Active Thyroid Eye Disease (TED) |
| 2. A multicenter, open-label, randomized, active-controlled, Phase 2 study to evaluate the pharmacokinetics, efficacy, and safety of intravenous BV100 combined with Polymyxin B versus best available therapy in adult patients with ventilator-associated bacterial pneumonia suspected or confirmed to be due to carbapenem-resistant Acinetobacter baumannii |
| 3. A Phase 3 Study of Relacorilant in Combination with Nab-Paclitaxel versus Nab-Paclitaxel Monotherapy in Advanced, Platinum-Resistant, High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian-Tube Cancer (ROSELLA) |
| 4. A Phase 3 Randomized Study Comparing Teclistamab in Combination with Daratumumab SC and Lenalidomide (Tec-DR) versus Daratumumab SC, Lenalidomide, and Dexamethasone (DRd) in Participants with Newly Diagnosed Multiple Myeloma Who are Either Ineligible or not Intended for Autologous Stem Cell Transplant as Initial Therapy |
| 5. A Phase III, Multicentre, Randomised, Double-blind, Parallel-group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Tozorakimab (MEDI3506) in Patients Hospitalised for Viral Lung Infection Requiring Supplemental Oxygen (TILIA) |
| 6. An 18-month, open-label, single-arm safety extension study of an age-and bodyweight-adjusted oral finerenone regimen, in addition to an ACEI or ARB, for the treatment of children and young adults from 1 to 18 years of age with chronic kidney disease and proteinuria |
| 7. A Phase 2, Randomized, Placebo-Controlled, Parallel-Group, Double-Blinded, Proof-of-Concept Study to Evaluate the Safety and Efficacy of Intravenous Efgartigimod in Adult Participants With Primary Sjögren’s Syndrome |
| 8. A randomized, double-blind, three-part, first-in-human study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of single ascending (SAD17663) and multiple ascending (MAD17664) doses of SAR444419 in healthy adult participants and of a single dose (PDY17062) of SAR444419 in adult participants with moderate-to-severe rheumatoid arthritis |
| 9. A Phase 2a, Randomized, Open-Label Study to Evaluate the Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of ISIS 702843 Administered to Patients with Phlebotomy Dependent Polycythemia Vera (PD-PV) |
| 10. A Phase 3, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study to Assess the Efficacy and Safety of Batoclimab as Induction and Maintenance Therapy in Adult Participants with Generalized Myasthenia Gravis (gMG) |
| 11. A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel, Multicenter Study to Evaluate the Safety and Efficacy of ALXN1720 in Adults with Generalized Myasthenia Gravis |
| 12. A phase 3, randomized, placebo-controlled, double-blind, multicenter trial of selinexor in maintenance therapy after systemic therapy for patients with p53 wild-type, advanced or recurrent endometrial carcinoma |
| 13. A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of IgPro20 (Subcutaneous Immunoglobulin, Hizentra®) in Adults with Dermatomyositis (DM) - The RECLAIIM Study |
| 14. PART I A Phase 3, Open-label, 52 week Study to Assess the Safety, Tolerability, and Efficacy of Rocatinlimab (AMG 451) in Adolescent Subjects Aged ≥ 12 to < 18 years With Moderate to severe Atopic Dermatitis (AD) (ROCKET-Orbit) |
| 15. PART II "A Phase 3, Open-label, 52 week Study to Assess the Safety, Tolerability, and Efficacy of Rocatinlimab (AMG 451) in Adolescent Subjects Aged ≥ 12 to < 18 years With Moderate to severe Atopic Dermatitis (AD) (ROCKET-Orbit) |
| 16. Phase 1, Open-label, Single-Dose Study to Determine the Effect of Hepatic Impairment on the Pharmacokinetics, Safety, and Tolerability of TL-895 in Adult Subjects |
| 17. Evaluation of the bioequivalence of two products containing aripiprazole: Aripiprazole LAI powder and solvent for prolonged-release suspension for intramuscular injection, 400mg, Greece (Test) vs. ABILIFY MAINTENA® (aripiprazole) for extended-release injectable suspension, for intramuscular use, 400mg, USA (Reference). A multicentric, multi-national, open-label, randomized, multiple dose, two-period, crossover pharmacokinetic study in schizophrenic patients |
| 18. A PHASE 2 RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED STUDY TO INVESTIGATE THE EFFICACY, SAFETY AND TOLERABILITY OF PONSEGROMAB IN PATIENTS WITH CANCER, CACHEXIA, AND ELEVATED CONCENTRATIONS OF GDF-15, FOLLOWED BY AN OPTIONAL OPEN-LABEL TREATMENT PERIOD |
| 19. Phase 1/2, Open-label, Dose Escalation and Dose Expansion Study of TransCon TLR7/8 Agonist Alone or in Combination with Pembrolizumab in Participants with Locally Advanced or Metastatic Solid Tumor Malignancies |
| 20. A Phase 1/2a Open-label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of Modakafusp Alfa in Combination With Daratumumab Subcutaneous in Patients With Relapsed or Refractory Multiple Myeloma |
| 21. An Open-label Extension Study of ARGX-113-2009 to Evaluate the Long-term Safety, Tolerability, and Efficacy of Efgartigimod PH20 SC in Adult Participants With Bullous Pemphigoid |
| 22. A Phase 3, Multicenter, Open-Label, Randomized Study of Nemvaleukin Alfa in Combination With Pembrolizumab Versus Investigator's Choice Chemotherapy in Patients With Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer (ARTISTRY-7) |
| 23. A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Guselkumab Subcutaneous Induction Therapy in Participants with Moderately to Severely Active Ulcerative Colitis |
| 24. Phase 3 Multicenter, Randomized, Double-Blind, Study to "Assess the Efficacy and Safety of Treatment with Bepirovirsen in HBeAg-negative Nucleos(t)ide Analogue-treated Participants with Chronic Hepatitis B Virus (B-Well 2) |
| 25. A multicenter rollover extension program (REP) to evaluate the long-term safety and tolerability of open label iptacopan in adult participants with primary IgA nephropathy who have completed study CLNP023X2203 or CLNP023A2301 |
| 26. Part I. "A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of Olezarsen (ISIS 678354) Administered Subcutaneously to Patients with Severe Hypertriglyceridemia |
| 27. Part II. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of Olezarsen (ISIS 678354) Administered Subcutaneously to Patients with Severe Hypertriglyceridemia |
| 28. Part I. A Phase III, Multicentre, Randomised, Double-blind, Chronic-dosing, Parallelgroup, Placebo-controlled Extension Study to Evaluate the Long-term Efficacy and Safety of Tozorakimab in Participants with Chronic Obstructive Pulmonary Disease (COPD) with a History of Exacerbations (PROSPERO) |
| 29. Part II. A Phase III, Multicentre, Randomised, Double-blind, Chronic-dosing, Parallelgroup, Placebo-controlled Extension Study to Evaluate the Long-term Efficacy and Safety of Tozorakimab in Participants with Chronic Obstructive Pulmonary Disease (COPD) with a History of Exacerbations (PROSPERO) |
| 30. Part I. A Phase 3, 24-week, Randomized, Placebo controlled, Double-blind Study to Assess the Efficacy, Safety and Tolerability of Rocatinlimab (AMG 451) Monotherapy in Adult Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET Ignite) |
| 31. Part II. A Phase 3, 24-week, Randomized, Placebo controlled, Double-blind Study to Assess the Efficacy, Safety and Tolerability of Rocatinlimab (AMG 451) Monotherapy in Adult Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET Ignite) |
| 32. Part I. A Randomized, Double-blind, Placebo-controlled, Multicenter Phase 2b/3 Study to Evaluate the Efficacy and Safety of Izokibep in Subjects with Active Psoriatic Arthritis |
| 33. Part II. A Randomized, Double-blind, Placebo-controlled, Multicenter Phase 2b/3 Study to Evaluate the Efficacy and Safety of Izokibep in Subjects with Active Psoriatic Arthritis |
| 34. A randomized, double-blind, placebo-controlled study to investigate the efficacy and safety of carisbamate (YKP509) as adjunctive treatment for seizures associated with Lennox-Gastaut Syndrome in children and adults, with optional open-label extension |
| 35. Phase 3 Multicenter, Randomized, Double-Blind, Study to Assess the Efficacy and Safety of Treatment with Bepirovirsen in HBeAg-negative Nucleos(t)ide Analogue-treated Participants with Chronic Hepatitis B Virus (B Well 1) |
| 36. A Multi-Cohort, Randomised, Placebo-Controlled Phase 2a Study to Assess the Safety, Pharmacokinetics, Pharmacodynamics and Clinical Activity of Ascending Doses of RXC007 in Patients with Idiopathic Pulmonary Fibrosis |
| 37. A Phase 2, Multicenter, Multi Arm, Study to Evaluate MK-1308A (Co-formulated quavonlimab (MK-1308)/pembrolizumab) Versus Other Treatments in Participants with Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Stage IV Colorectal Cancer: (MK-1308A-008) |
| 38. An Open-label Randomized Phase 3 Study of Tucatinib in Combination with Trastuzumab and mFOLFOX6 versus mFOLFOX6 given with or without either Cetuximab or Bevacizumab as First-line Treatment for Subjects with HER2+ Metastatic Colorectal Cancer |
| 39. A Phase 3, Randomized, Double-Blind, Parallel-Group, Multicenter Study to Compare Efficacy, Safety, Pharmacodynamics, Pharmacokinetics, and Immunogenicity of BP11 Versus EU-Approved Xolair® in Patients With Chronic Spontaneous Urticaria Who Are Resistant to H1 Antagonist |
| 40. An Open-label Extension Study for Participants who Completed Study IMVT-1401-3201 or Study IMVT-1401-3202 to Assess the Efficacy and Safety of Batoclimab for the Treatment of Thyroid Eye Disease (TED) |
| 41. A Randomized, Double-masked, Parallel-group, Multicenter Clinical Study to Evaluate the Efficacy and Safety of AVT06 Compared with EU-Eylea® in Subjects with Neovascular (wet) Age related Macular Degeneration (ALVOEYE) |
| 42. A Phase 2 Study Evaluating the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Narsoplimab in Paediatric Patients (28 Days to 18 Y.O.) with High Risk Haematopoietic Stem Cell Transplant Thrombotic Microangiopathy |
| 43. PART I A Randomized Phase 3 Study of Datopotamab Deruxtecan (Dato-DXd) and Pembrolizumab, with or Without Platinum Chemotherapy, in Subjects with No Prior Therapy for Advanced or Metastatic PD-L1 TPS <50% Non-squamous Non-small Cell Lung Cancer Without Actionable Genomic Alterations (TROPION-Lung07) |
| 44. PART II A Randomized Phase 3 Study of Datopotamab Deruxtecan (Dato-DXd) and Pembrolizumab, with or Without Platinum Chemotherapy, in Subjects with No Prior Therapy for Advanced or Metastatic PD-L1 TPS <50% Non-squamous Non-small Cell Lung Cancer Without Actionable Genomic Alterations (TROPION-Lung07) |
| 45. Phase I/II multicenter study to assess efficacy and safety of ribociclib (LEE011) in combination with topotecan and temozolomide (TOTEM) in pediatric patients with relapsed or refractory neuroblastoma and other solid tumors |
| 46. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Zilovertamab (an ROR1 Antibody) Plus Ibrutinib Versus Ibrutinib Plus Placebo in Subjects with Relapsed or Refractory Mantle Cell Lymphoma |
| 47. A Phase IIb/III Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of Cotadutide in Participants with Non-cirrhotic Non-alcoholic Steatohepatitis with Fibrosis |
| 48. A Phase 2 Long-Term Extension (LTE) Study to Evaluate The Safety and Efficacy of Efavaleukin Alfa in Subjects with Moderately to Severely Active Ulcerative Colitis |
| 49. Open-label, multicenter, multinational, interventional Clinical Trial to assess Efficacy and Safety of the extemporaneous combination of Nebivolol and Ramipril in hypertensive patients - ARTEMISIA study |
| 50. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Dose ranging Study to Assess the Efficacy and Safety of CDX-0159 in Patients with Chronic Inducible Urticaria |
| 51. A Phase 3 Trial of the Efficacy and Safety of Bardoxolone Methyl in Patients with Autosomal Dominant Polycystic Kidney Disease |
| 52. An Open-Label Extension Study to Evaluate the Long-Term Safety, Tolerability, and Efficacy of Pozelimab and Cemdisiran Combination Therapy in Patients with Paroxysmal Nocturnal Hemoglobinuria |
| 53. Phase IV study comparing the efficacy and safety of an alcohol-free formulation of 0.15% benzydamine hydrochloride spray and benzydamine hydrochloride 3mg lozenges in paediatric patients (6-12 years) with sore throat |
| 54. A 24-Week, Phase 2b, Randomized, Double-Blind Long-Term Extension Study to Evaluate Pharmacokinetics, Efficacy Safety, and Tolerability of TEV-48574 in Adult Patients with Moderate to Severe Ulcerative Colitis or Crohn's Disease who completed the treatment phase of the Dose-Ranging Study |
| 55. A 20-Week Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of EPX-100 (Clemizole HCl) as Adjunctive Therapy in Patients with Dravet Syndrome (ARGUS trial) |
| 56. A Phase 3 Randomized Study of Loncastuximab Tesirine Combined with Rituximab Versus Immunochemotherapy in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL) (LOTIS-5) |
| 57. A Randomized, Double-blind, Placebo-controlled Study Evaluating the Safety and Efficacy of Guselkumab for the Treatment of Participants with Crohn’s Disease After Surgical Resection |
| 58. A Global, Phase 3, Randomized, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Furmonertinib Compared to Platinum-Based Chemotherapy as First-Line Treatment for Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Exon 20 Insertion Mutations |
| 59. A Phase 1b/2 Study of Immune and Targeted Combination Therapies in Participants With RCC (KEYMAKER-U03): Substudy 03B |
| 60. Part I A Phase 3 Randomized, Open-label Clinical Study to Evaluate the Pharmacokinetics and Safety of Subcutaneous Pembrolizumab Coformulated With Hyaluronidase (MK-3475A) Versus Intravenous Pembrolizumab, Administered With Chemotherapy, in the First-line Treatment of Participants With Metastatic Non-small Cell Lung Cancer |
| 61. PART II A Phase 3 Randomized, Open-label Clinical Study to Evaluate the Pharmacokinetics and Safety of Subcutaneous Pembrolizumab Coformulated With Hyaluronidase (MK-3475A) Versus Intravenous Pembrolizumab, Administered With Chemotherapy, in the First-line Treatment of Participants With Metastatic Non-small Cell Lung Cancer |
| 62. A Phase 1b/2 Study of Immune and Targeted Combination Therapies in Participants with RCC (KEYMAKER-U03): Substudy 03A |
| 63. Multi-center, Randomized, Double-blind, Parallel-group, Double-dummy, Active-controlled, Comparative Study to Evaluate the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Ponesimod Versus Fingolimod During 108 Weeks of Treatment in Pediatric Participants, 10 to <18 Years Old, with Relapsing-remitting Multiple Sclerosis |
| 64. A Phase 2b, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study of Batoclimab Treatment in Adult Participants with Active Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) |
| 65. A Prospective, Multi-Centre Study (B-Sure) to Evaluate Long-Term Durability of Sustained Virologic Response in Chronic Hepatitis B Participants With and Without Nucleos(t)ide Therapy Who Have Received and Responded to GSK3228836 in a Previous Treatment Study |
| 66. A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of VTX958 in Participants with Moderately to Severely Active Crohn’s Disease |
| 67. An open-label extension trial to evaluate the long-term safety of apraglutide in short bowel syndrome |
| 68. An Open-Label, 2-Arm, Multicenter, Randomized Phase 3 Study To Evaluate The Efficacy And Safety of Elranatamab (PF-06863135) + Daratumumab + Lenalidomide Versus Daratumumab + Lenalidomide + Dexamethasone in Transplant-Ineligible Participants With Newly-Diagnosed Multiple Myeloma |
| 69. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Pharmacodynamics and Pharmacokinetics of TW001 in Alzheimer Patients |
| 70. An Integrated Phase I/II, Multicentre, Double-Blind, Randomised, Dysport and Placebo-Controlled, Dose-Escalation and Dose-Finding Study to Evaluate the Safety and Efficacy of IPN10200 in the Treatment of Adult Upper Limb Spasticity |
| 71. An open-label, parallel-group study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of cenerimod after single-dose administration in subjects with hepatic impairment and in healthy subjects |
| 72. A multicenter, open-label, Phase IIb study to evaluate the efficacy, safety and pharmacokinetics of rilzabrutinib in patients with warm autoimmune hemolytic anemia |
| 73. A Phase 1, open-label study to investigate the pharmacokinetics and safety of remibrutinib (LOU064) in participants with renal impairment compared to matched healthy participants with normal renal function |
| 74. A First-in-Human, Multicenter, Phase 1/2, Open-Label Study of XTX202 in Patients with Advanced Solid Tumors |
| 75. Phase 1b/2a safety and tolerability study of bemcentinib with pembrolizumab/carboplatin/pemetrexed in subjects with untreated advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) without/with a STK11 mutation |
| 76. A Phase I Dose Escalation Study of NMS-03305293 in Adult Patients with Selected Advanced/Metastatic Solid Tumors |
| 77. Performance of Elucirem® (gadopiclenol) in Dynamic Susceptibility Contrast Magnetic Resonance Imaging (DSC-MRI) perfusion of brain gliomas |
| 78. A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of VTX958 in Patients with Active Psoriatic Arthritis |
| 79. A Randomized, Double-blind, Placebo-controlled, Phase 3 Study of Pembrolizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy for the Treatment of Chemotherapy-Candidate Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative (HR+/HER2-) Locally Recurrent Inoperable or Metastatic Breast Cancer (KEYNOTE-B49) |
| 80. Part I A Phase 1 Study to Investigate the Absolute Bioavailability and Absorption, Metabolism, and Excretion of 14C-Unesbulin in Patients With Advanced Solid Tumor |
| 81. Part II A Phase 1 Study to Investigate the Absolute Bioavailability and Absorption, Metabolism, and Excretion of 14C-Unesbulin in Patients With Advanced Solid Tumor |
| 82. PART I A Randomized, Double-Blind, Placebo-Controlled Multicenter Study To Evaluate The Efficacy And Safety Of CAL02 Administered Intravenously In Addition To Standard Of Care In Subjects With Severe Community-Acquired Bacterial Pneumonia (SCABP) |
| 83. Randomized, double-blind, placebo controlled clinical trial of (+)-?-dihydrotetrabenazine in patients with moderate to severe tardive dyskinesia |
| 84. PART I A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obexelimab in Patients with IgG4-Related Disease (INDIGO) |
| 85. PART II A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obexelimab in Patients with IgG4-Related Disease (INDIGO) |
| 86. Phase 1/2, Open label & double blind randomized placebo-controlled study to assess the feasibility of BGC101 (EnEPC) in the treatment of peripheral arterial disease (PAD) with critical limb ischemia (CLI) |
| 87. PANOVA-4: Pilot, Single arm Study of Tumor Treating Fields (TTFields, 150kHz) Concomitant with Atezolizumab, Gemcitabine and Nab-Paclitaxel as First-Line Treatment for Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC) |
| 88. PART I A Phase 3, Randomized, 24-week, Placebo controlled, Double-blind Study to Assess the Efficacy, Safety, and Tolerability of Rocatinlimab (AMG 451) in Combination With Topical Corticosteroids and/or Topical Calcineurin Inhibitors in Adult Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET SHUTTLE) |
| 89. PART II A Phase 3, Randomized, 24-week, Placebo controlled, Double-blind Study to Assess the Efficacy, Safety, and Tolerability of Rocatinlimab (AMG 451) in Combination With Topical Corticosteroids and/or Topical Calcineurin Inhibitors in Adult Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET SHUTTLE) |
| 90. PART I A Phase 3, Randomized, 52-week, Placebo-controlled, Double-blind Study With Rerandomization to Assess the Efficacy, Safety, and Tolerability of Rocatinlimab (AMG 451) in Adolescent Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET-ASTRO) |
| 91. PART II A Phase 3, Randomized, 52-week, Placebo-controlled, Double-blind Study With Rerandomization to Assess the Efficacy, Safety, and Tolerability of Rocatinlimab (AMG 451) in Adolescent Subjects With Moderate-to-severe Atopic Dermatitis (AD) (ROCKET-ASTRO) |
| 92. PART I A parallel-group treatment, proof-of-concept Phase 2, multicenter, double-blind, randomized two-arm clinical trial to investigate the efficacy and safety of subcutaneous NT 201 injections compared with placebo injections in decreasing pain intensity in male and female participants aged 18 years and older with moderate to severe chronic peripheral neuropathic pain due to postherpetic neuralgia or peripheral nerve injury. |
| 93. PART II A parallel-group treatment, proof-of-concept Phase 2, multicenter, double-blind, randomized two-arm clinical trial to investigate the efficacy and safety of subcutaneous NT 201 injections compared with placebo injections in decreasing pain intensity in male and female participants aged 18 years and older with moderate to severe chronic peripheral neuropathic pain due to postherpetic neuralgia or peripheral nerve injury |
| 94. PART I A randomized, double-blind, placebo controlled, dose-finding study to assess the efficacy and safety of SAR443122 in adult patients with moderate to severe ulcerative colitis |
| 95. PART II A randomized, double-blind, placebo controlled, dose-finding study to assess the efficacy and safety of SAR443122 in adult patients with moderate to severe ulcerative colitis |
| 96. A Randomized, Double masked, Parallel group, Dose-finding study to evaluate SYL1801 in patients with neovascular AMD |
| 97. Part I A Phase 2, Double-Blinded, Randomized, Placebo- Controlled, Dose-Ranging Study Evaluating the Efficacy and Safety of GS.5290 in Participants With Moderately to Severely Active Ulcerative Colitis |
| 98. Part II A Phase 2, Double-Blinded, Randomized, Placebo- Controlled, Dose-Ranging Study Evaluating the Efficacy and Safety of GS.5290 in Participants With Moderately to Severely Active Ulcerative Colitis |
| 99. Part I A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Deucravacitinib in Participants with Active Systemic Lupus Erythematosus (SLE) (POETYK SLE-2) |
| 100. Part II A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Deucravacitinib in Participants with Active Systemic Lupus Erythematosus (SLE) (POETYK SLE-2) |
| 101. Part I A Phase 3, Open Label, Randomized, Two Part Study Comparing Gedatolisib in Combination with Palbociclib and Fulvestrant to Standard of Care Therapies in Patients with HR Positive, HER2 Negative Advanced Breast Cancer Previously Treated with a CDK4/6 Inhibitor in Combination with Non-Steroidal Aromatase Inhibitor Therapy (VIKTORIA-1) |
| 102. A Phase 3, Open Label, Randomized, Two Part Study Comparing Gedatolisib in Combination with Palbociclib and Fulvestrant to Standard of Care Therapies in Patients with HR Positive, HER2 Negative Advanced Breast Cancer Previously Treated with a CDK4/6 Inhibitor in Combination with Non-Steroidal Aromatase Inhibitor Therapy (VIKTORIA-1) |
| 103. Part I A Phase 2b / 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Combined Dose-Finding and Cardiovascular Outcome Study to Investigate the Efficacy and Safety of CSL300 (Clazakizumab) in Subjects with End Stage Kidney Disease Undergoing Dialysis |
| 104. A Phase 2b / 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Combined Dose-Finding and Cardiovascular Outcome Study to Investigate the Efficacy and Safety of CSL300 (Clazakizumab) in Subjects with End Stage Kidney Disease Undergoing Dialysis |
| 105. A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants With Melanoma (KEYMAKER-U02): Substudy 02B |
| 106. A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants With Melanoma (KEYMAKER-U02): Substudy 02D |
| 107. Changes in airway cross sectional areas during residual neuromuscular blockade, and after reversal |
| 108. The effect of additional pre-extubational loading dose of caffeine-citrate |
| 109. A First-in-Human, Open-label, Phase 1/2 Study to Evaluate the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Activity of JNJ-63723283, an Anti-PD-1 Monoclonal Antibody, in Subjects with Advanced Cancers |
| 110. A Multicenter, Randomized, Double-Blind, Parallel-Controlled Phase III Clinical Study to Evaluate the Efficacy and Safety of Pertuzumab Biosimilar HLX11 vs. EU-Perjeta® in the Neoadjuvant Therapy of HER2-Positive and HR-Negative Early-stage or Locally Advanced Breast Cancer |
| 111. A MULTICENTER, RANDOMIZED, OPEN-LABEL, ADAPTIVE PHASE I STUDY TO INVESTIGATE THE SAFETY, TOLERABILITY, IMMUNOGENICITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF ASCENDING INTRAVENOUS DOSES OF GLOFITAMAB, ADMINISTERED AFTER OCRELIZUMAB PRETREATMENT, IN PATIENTS WITH MULTIPLE SCLEROSIS |
| 112. Roll Over StudY for Patients Who Have Completed a Previous Oncology Study with Durvalumab and Are Judged by the Investigator to Clinically Benefit From Continued Treatment |
| 113. A Phase 3b Study to Evaluate Higher Dose Nusinersen (BIIB058) in Patients with Spinal Muscular Atrophy Previously Treated with Risdiplam |
| 114. PART I A Phase 3, Randomized, Open-label, Multicenter Trial of ARV-471 (PF-07850327) vs Fulvestrant in Participants With Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer whose disease progressed after prior endocrine based treatment for advanced disease (VERITAC-2) |
| 115. PART II A Phase 3, Randomized, Open-label, Multicenter Trial of ARV-471 (PF-07850327) vs Fulvestrant in Participants With Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer whose disease progressed after prior endocrine based treatment for advanced disease (VERITAC-2) |
| 116. PART I HERMES: Effects of ziltivekimab versus placebo on morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction and systemic inflammation |
| 117. PART II HERMES: Effects of ziltivekimab versus placebo on morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction and systemic inflammation |
| 118. Individualized everolimus therapy in metastatic breast cancer |
| 119. Phase 2, Multicenter, Randomized, Parallel, 3-arm, Placebo-controlled Study to Assess Efficacy and Safety of CDR132L in Patients with Reduced Left Ventricular Ejection Fraction ( ˂ 45%) After Myocardial Infarction (HF-REVERT) |
| 120. Implementation study of lipid management of high-risk cardiovascular patients- Semmelweis Lipid Center for high-risk patients |
| 121. Value-based health economic comparative study of subcutaneous and intravenous trastuzumab-pertuzumab for HER-2 positive metastatic breast cancer based on patient reported outcomes |
| 122. PART I A Phase 3 randomized, placebo-controlled, double-blind, parallelgroup program to evaluate efficacy and safety of filgotinib in adult subjects with active axial spondyloarthritis |
| 123. PART II A Phase 3 randomized, placebo-controlled, double-blind, parallelgroup program to evaluate efficacy and safety of filgotinib in adult subjects with active axial spondyloarthritis |
| 124. PART I A Randomized, Double-blind, Placebo-Controlled, Phase 3 Study of Olezarsen (ISIS 678354) in Patients with Hypertriglyceridemia and Atherosclerotic Cardiovascular Disease (Established or at Increased Risk for), or with Severe Hypertriglyceridemia |
| 125. PART I A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Olpasiran on Major Cardiovascular Events in Patients with Atherosclerotic Cardiovascular Disease and Elevated Lipoprotein (a) |
| 126. PART II A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Olpasiran on Major Cardiovascular Events in Patients with Atherosclerotic Cardiovascular Disease and Elevated Lipoprotein (a) |
| 127. PART II A Randomized, Double-blind, Placebo-Controlled, Phase 3 Study of Olezarsen (ISIS 678354) in Patients with Hypertriglyceridemia and Atherosclerotic Cardiovascular Disease (Established or at Increased Risk for), or with Severe Hypertriglyceridemia |
| 128. PART I A Multi-Center, Parallel, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate Efficacy, Safety, and Pharmacokinetics of XEMBIFY® plus Standard Medical Treatment Compared to Placebo plus Standard Medical Treatment to Prevent Infections in Patients with Hypogammaglobulinemia and Recurrent or Severe Infections Associated with B-cell Chronic Lymphocytic Leukemia |
| 129. PART I An Open-Label Extension Study of Olezarsen (ISIS 678354) Administered Subcutaneously to Patients with Severe Hypertriglyceridemia (SHTG) |
| 130. PART II An Open-Label Extension Study of Olezarsen (ISIS 678354) Administered Subcutaneously to Patients with Severe Hypertriglyceridemia (SHTG) |
| 131. PART I A Randomized, Double-Blind, Placebo-Controlled Study of Trilaciclib vs Placebo in Patients with Extensive Stage Small Cell Lung Cancer (ES-SCLC) Receiving Topotecan Chemotherapy |
| 132. PART II A Randomized, Double-Blind, Placebo-Controlled Study of Trilaciclib vs Placebo in Patients with Extensive Stage Small Cell Lung Cancer (ES-SCLC) Receiving Topotecan Chemotherapy |
| 133. PART I A Randomized, Double-blind, Placebo-Controlled, Multicenter Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Focal-Onset Seizures (X-TOLE3) |
| 134. PART II A Randomized, Double-blind, Placebo-Controlled, Multicenter Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Focal-Onset Seizures (X-TOLE3) |
| 135. PART I Double-blind, randomized, active-controlled, two-way cross-over study, with 12-week treatment duration per period, to evaluate the efficacy and safety of QMF149 (indacaterol acetate / mometasone furoate) compared to budesonide in children from 6 to less than 12 years of age with asthma |
| 136. PART II Double-blind, randomized, active-controlled, two-way cross-over study, with 12-week treatment duration per period, to evaluate the efficacy and safety of QMF149 (indacaterol acetate / mometasone furoate) compared to budesonide in children from 6 to less than 12 years of age with asthma |
| 137. A comparative bioavailability study of a single dose of ziltivekimab formulation B in a manual syringe, formulation D in a manual syringe and formulation C in a pen-injector |
| 138. PART II A randomized, placebo-controlled, double-blind, multi-center, phase III trial to assess the efficacy and safety of trimodulin (BT588) in adult hospitalized subjects with severe community-acquired pneumonia (sCAP |
| 139. PART I A Multicenter, Double-Blind, Placebo-Controlled, Randomized Withdrawal Study to Evaluate the Safety and Maintenance of Efficacy of Ecopipam in Children, Adolescents and Adults with Tourette’s Disorder |
| 140. PART II A Multicenter, Double-Blind, Placebo-Controlled, Randomized Withdrawal Study to Evaluate the Safety and Maintenance of Efficacy of Ecopipam in Children, Adolescents and Adults with Tourette’s Disorder |
| 141. PART I A Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaLuate the effIcacy and safety of abeLacimab in high-risk patients with Atrial fibrillation who have been deemed unsuitable for oral antiCoagulation (LILAC) |
| 142. PART II A Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaLuate the effIcacy and safety of abeLacimab in high-risk patients with Atrial fibrillation who have been deemed unsuitable for oral antiCoagulation (LILAC) |
| 143. PART I An 18-month low-interventional prospective, multicentre study to assess joint outcomes in patients with haemophilia A or B on prophylaxis with efmoroctocog alfa or eftrenonacog alfa JOIN-us |
| 144. PART II An 18-month low-interventional prospective, multicentre study to assess joint outcomes in patients with haemophilia A or B on prophylaxis with efmoroctocog alfa or eftrenonacog alfa JOIN-us |
| 145. PART I A randomized, parallel-group, double-blind, placebocontrolled, multicenter Phase III trial to evaluate efficacy and safety of secukinumab administered subcutaneously versus placebo, in combination with a glucocorticoid taper regimen, in patients with polymyalgia rheumatica (PMR) |
| 146. PART II A randomized, parallel-group, double-blind, placebocontrolled, multicenter Phase III trial to evaluate efficacy and safety of secukinumab administered subcutaneously versus placebo, in combination with a glucocorticoid taper regimen, in patients with polymyalgia rheumatica (PMR) |
| 147. PART I A randomized, double-blind, multicenter phase 3 study in patients with moderately to severely active ulcerative colitis (UC) to compare the efficacy, safety and immunogenicity of PB016 and Entyvio® for the induction and maintenance of clinical response and remission. (UCESIVE) |
| 148. PART II A randomized, double-blind, multicenter phase 3 study in patients with moderately to severely active ulcerative colitis (UC) to compare the efficacy, safety and immunogenicity of PB016 and Entyvio® for the induction and maintenance of clinical response and remission. (UCESIVE) |
| 149. PART I A Phase l/2a, Open-label, Dose-finding Trial to Evaluate Safety, Immunogenicity, and Anti-tumor Activity ofVBl0.16 in Combination with Pembrolizumab in Patients with Umesectable Recurrent or Metastatic HPV16-positive Head and Neck Squamous Cell Carcinoma |
| 150. PART II A Phase l/2a, Open-label, Dose-finding Trial to Evaluate Safety, Immunogenicity, and Anti-tumor Activity ofVBl0.16 in Combination with Pembrolizumab in Patients with Umesectable Recurrent or Metastatic HPV16-positive Head and Neck Squamous Cell Carcinoma |
| 151. PART I A PHASE 1/2, OPEN-LABEL, MULTI-CENTER STUDY OF ZN-c3 ADMINISTERED IN COMBINATION WITH ENCORAFENIB AND CETUXIMAB IN ADULTS WITH METASTATIC COLORECTAL CANCER |
| 152. PART I A PHASE 1/2, OPEN-LABEL, MULTI-CENTER STUDY OF ZN-c3 ADMINISTERED IN COMBINATION WITH ENCORAFENIB AND CETUXIMAB IN ADULTS WITH METASTATIC COLORECTAL CANCER |
| 153. PART I A Randomized, Double-blind, Placebo-Controlled, Multicenter, Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Primary Generalized Tonic-Clonic Seizures (X-ACKT) |
| 154. PART I A Phase 2b, Randomized, Double-masked, Multicenter, Dose-ranging, Sham-controlled Clinical Trial to Evaluate Intravitreal JNJ-81201887(AAVCAGsCD59) Compared to Sham procedure for the Treatment of Geographic Atrophy (GA) Secondary to Age-related Macular Degeneration (AMD) |
| 155. PART II A Phase 2b, Randomized, Double-masked, Multicenter, Dose-ranging, Sham-controlled Clinical Trial to Evaluate Intravitreal JNJ-81201887(AAVCAGsCD59) Compared to Sham procedure for the Treatment of Geographic Atrophy (GA) Secondary to Age-related Macular Degeneration (AMD) |
| 156. PART I A Randomized Open-Label Phase 3 Study of XL092 + Nivolumab vs Sunitinib in Subjects with Advanced or Metastatic Non-Clear Cell Renal Cell Carcinoma |
| 157. PART II A Randomized Open-Label Phase 3 Study of XL092 + Nivolumab vs Sunitinib in Subjects with Advanced or Metastatic Non-Clear Cell Renal Cell Carcinoma |
| 158. Phase 2b, multinational, randomized, double-blind study to investigate the efficacy and safety of redasemtide (S-005151) compared with placebo in adult participants with acute ischemic stroke who are not eligible for tissue plasminogen activator or thrombectomy Part I |
| 159. Phase 2b, multinational, randomized, double-blind study to investigate the efficacy and safety of redasemtide (S-005151) compared with placebo in adult participants with acute ischemic stroke who are not eligible for tissue plasminogen activator or thrombectomy Part II |
| 160. A phase III multicenter, double-blind, randomized, placebo-controlled, parallel-group trial of the efficacy of citicoline eye drops 2% on visual field preservation in patients with open angle glaucoma Part I |
| 161. A phase III multicenter, double-blind, randomized, placebo-controlled, parallel-group trial of the efficacy of citicoline eye drops 2% on visual field preservation in patients with open angle glaucoma Part II |
| 162. A Phase 3, Randomized, Double-blind, Placebo-controlled, Event-driven Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, After a Recent Acute Coronary Syndrome - LIBREXIA-ACS Part I |
| 163. A Phase 3, Randomized, Double-blind, Placebo-controlled, Event-driven Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, After a Recent Acute Coronary Syndrome - LIBREXIA-ACS Part II |
| 164. A Phase 3, Randomized, Double-Blind, Double-Dummy, Parallel Group, Active-Controlled Study to Evaluate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, Versus Apixaban in Participants with Atrial Fibrillation Part I |
| 165. A Phase 3, Randomized, Double-Blind, Double-Dummy, Parallel Group, Active-Controlled Study to Evaluate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, Versus Apixaban in Participants with Atrial Fibrillation Part II |
| 166. A Phase 3 multiCenter, rAndomized, pLacebo-controlled, double-blind studY to evaluate the efficacy and safety of eneboparatide (AZP-3601), a Parathyroid hormone receptor agoniSt, in patients with chronic hypOparathyroidism (CALYPSO) Part I |
| 167. A Phase 3 multiCenter, rAndomized, pLacebo-controlled, double-blind studY to evaluate the efficacy and safety of eneboparatide (AZP-3601), a Parathyroid hormone receptor agoniSt, in patients with chronic hypOparathyroidism (CALYPSO) Part II |
| 168. A Phase 2/3 Study of Navtemadlin as Maintenance Therapy in Subjects with TP53WT Advanced or Recurrent Endometrial Cancer Who Responded to Chemotherapy Part I |
| 169. A Phase 2/3 Study of Navtemadlin as Maintenance Therapy in Subjects with TP53WT Advanced or Recurrent Endometrial Cancer Who Responded to Chemotherapy Part II |
| 170. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study To Evaluate The Efficacy And Safety Of CAL02 Administered Intravenously In Addition To Standard Of Care In Subjects With Severe Community-Acquired Bacterial Pneumonia (SCABP) Part II |
| 171. Interfant-21: International collaborative treatment protocol for infants under one year with KMT2A-rearranged acute lymphoblastic leukemia or mixed phenotype acute leukemia Part I |
| 172. Interfant-21: International collaborative treatment protocol for infants under one year with KMT2A-rearranged acute lymphoblastic leukemia or mixed phenotype acute leukemia Part II |
| 173. A Phase 3, Multicenter, 12-Week, Double Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Atogepant for the Preventive Treatment of Episodic Migraine in Pediatric Subjects 6 to 17 years of age Part I |
| 174. A Phase 3, Multicenter, 12-Week, Double Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Atogepant for the Preventive Treatment of Episodic Migraine in Pediatric Subjects 6 to 17 years of age Part II |
| 175. A TWO-COHORT, PHASE II, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PARALLEL-GROUP, PLACEBO-CONTROLLED STUDY EVALUATING THE EFFICACY AND SAFETY OF VIXARELIMAB COMPARED WITH PLACEBO IN PATIENTS WITH IDIOPATHIC PULMONARY FIBROSIS AND IN PATIENTS WITH SYSTEMIC SCLEROSIS.ASSOCIATED INTERSTITIAL LUNG DISEASE Part I |
| 176. A TWO-COHORT, PHASE II, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PARALLEL-GROUP, PLACEBO-CONTROLLED STUDY EVALUATING THE EFFICACY AND SAFETY OF VIXARELIMAB COMPARED WITH PLACEBO IN PATIENTS WITH IDIOPATHIC PULMONARY FIBROSIS AND IN PATIENTS WITH SYSTEMIC SCLEROSIS.ASSOCIATED INTERSTITIAL LUNG DISEASE Part II |
| 177. A Multicenter, Open-Label, Study to Evaluate the Long-term Safety of Ecopipam Tablets in Children, Adolescents and Adults with Tourette’s Disorder Part I |
| 178. A Multicenter, Open-Label, Study to Evaluate the Long-term Safety of Ecopipam Tablets in Children, Adolescents and Adults with Tourette’s Disorder Part II |
| 179. First-in-human, open-label Phase 1/2 study to investigate safety and efficacy of SAR445514, an NK-cell engager (NKCE) targeting B-cell maturation antigen (BCMA) in monotherapy in participants with relapsed/refractory multiple myeloma (RRMM) and in relapsed/refractory lightchain amyloidosis (RRLCA) PART I |
| 180. First-in-human, open-label Phase 1/2 study to investigate safety and efficacy of SAR445514, an NK-cell engager (NKCE) targeting B-cell maturation antigen (BCMA) in monotherapy in participants with relapsed/refractory multiple myeloma (RRMM) and in relapsed/refractory lightchain amyloidosis (RRLCA) PART II |
| 181. A Randomized, Open-label, Phase 3 Study of Tarlatamab Compared With Standard of Care in Subjects With Relapsed Small Cell Lung Cancer After Platinum-based First-line Chemotherapy (DeLLphi-304) PART I |
| 182. A Randomized, Open-label, Phase 3 Study of Tarlatamab Compared With Standard of Care in Subjects With Relapsed Small Cell Lung Cancer After Platinum-based First-line Chemotherapy (DeLLphi-304) PART II |
| 183. CAMBRIA-1: A Phase III, Open-Label, Randomised Study to Assess the Efficacy and Safety of Extended Therapy with Camizestrant (AZD9833, a Next Generation, Oral Selective Estrogen Receptor Degrader) versus Standard Endocrine Therapy (Aromatase Inhibitor or Tamoxifen) in Patients with ER+/HER2- Early Breast Cancer and an Intermediate or High Risk of Recurrence Who Have Completed Definitive Locoregional Therapy and at Least 2 Years of Standard Adjuvant Endocrine-Based Therapy Without Disease Recurrence PART I |
| 184. CAMBRIA-1: A Phase III, Open-Label, Randomised Study to Assess the Efficacy and Safety of Extended Therapy with Camizestrant (AZD9833, a Next Generation, Oral Selective Estrogen Receptor Degrader) versus Standard Endocrine Therapy (Aromatase Inhibitor or Tamoxifen) in Patients with ER+/HER2- Early Breast Cancer and an Intermediate or High Risk of Recurrence Who Have Completed Definitive Locoregional Therapy and at Least 2 Years of Standard Adjuvant Endocrine-Based Therapy Without Disease Recurrence PART II |
| 185. A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF ASTEGOLIMAB IN PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE PART I |
| 186. A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF ASTEGOLIMAB IN PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE PART II |
| 187. A Phase 3, Randomized, Double-Blind, Parallel- Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor Xia Inhibitor, for Stroke Prevention after an Acute lschemic Stroke or High-Risk Transient lschemic Attack PART I |
| 188. A Phase 3, Randomized, Double-Blind, Parallel- Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor Xia Inhibitor, for Stroke Prevention after an Acute lschemic Stroke or High-Risk Transient lschemic Attack PART II |
| 189. A Phase 2a/b, Randomized, Double-blind, Placebo-controlled, Parallel Group, Multicenter, Clinical Study to Evaluate the Efficacy and Safety of OG-6219 in 3 Dose Levels, in Women 18 to 49 Years of Age with Moderate to Severe Endometriosis-related Pain PART II |
| 190. A pivotal, multicentre, randomised, open-label, parallel-group trial on contraceptive efficacy, safety and tolerability of LVDS (Levonorgestrel Vaginal Delivery System) during 9 cycles in comparison with Desogestrel tablets 0.075mg PART I |
| 191. A pivotal, multicentre, randomised, open-label, parallel-group trial on contraceptive efficacy, safety and tolerability of LVDS (Levonorgestrel Vaginal Delivery System) during 9 cycles in comparison with Desogestrel tablets 0.075mg PART II |
| 192. A multicentre, single arm trial on contraceptive efficacy, safety and tolerability of LVDS (Levonorgestrel Vaginal Delivery System) during 13 cycles. PART I |
| 193. A multicentre, single arm trial on contraceptive efficacy, safety and tolerability of LVDS (Levonorgestrel Vaginal Delivery System) during 13 cycles PART II |
| 194. A Phase 1, Open-label, Single-dose Study to Evaluate the Pharmacokinetics of Mavodelpar in Subjects with Impaired Hepatic Function PART I |
| 195. A Phase 1, Open-label, Single-dose Study to Evaluate the Pharmacokinetics of Mavodelpar in Subjects with Impaired Hepatic Function PART II |
| 196. A Multicenter, Phase 3b, Open-Label, Single-Arm Study to Investigate Bowel Urgency and its Relationship with Other Outcome Measures in Adults with Moderately to Severely Active Ulcerative Colitis Treated with Mirikizumab PART I |
| 197. A Multicenter, Phase 3b, Open-Label, Single-Arm Study to Investigate Bowel Urgency and its Relationship with Other Outcome Measures in Adults with Moderately to Severely Active Ulcerative Colitis Treated with Mirikizumab PART II |
| 198. A Phase 2, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, 52-Week Study to Evaluate the Efficacy and Safety of LY3454738 in the Treatment of Adult Patients with Moderate-to-Severe Atopic Dermatitis part I |
| 199. A Phase 2, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, 52-Week Study to Evaluate the Efficacy and Safety of LY3454738 in the Treatment of Adult Patients with Moderate-to-Severe Atopic Dermatitis part II |
| 200. A Multi-Center, Randomized, Parallel -Group, Phase 2, Masked, Three -Arm Trial to Compare Safety, Tolerability, Efficacy, and Durability of Two Dose Levels of Suprachoroidal Sustained-Release OXU-001 (Dexamethasone Microspheres; DEXAspheres®) Using the Oxulumis® Illuminated Microcatheterization Device Compared with Intravitreal Dexamethasone Implant (OZURDEX®) in Subjects with Diabetic Macular Edema (OXEYE) part I |
| 201. A Multi-Center, Randomized, Parallel -Group, Phase 2, Masked, Three -Arm Trial to Compare Safety, Tolerability, Efficacy, and Durability of Two Dose Levels of Suprachoroidal Sustained-Release OXU-001 (Dexamethasone Microspheres; DEXAspheres®) Using the Oxulumis® Illuminated Microcatheterization Device Compared with Intravitreal Dexamethasone Implant (OZURDEX®) in Subjects with Diabetic Macular Edema (OXEYE) part II |
| 202. Randomized, Double-Masked, Active-Controlled, Phase 3 Study of the Efficacy and Safety of Aflibercept 8 mg in Macular Edema Secondary to Retinal Vein Occlusion part I |
| 203. Randomized, Double-Masked, Active-Controlled, Phase 3 Study of the Efficacy and Safety of Aflibercept 8 mg in Macular Edema Secondary to Retinal Vein Occlusion part II |
| 204. A Multi-Center, Parallel, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate Efficacy, Safety, and Pharmacokinetics of XEMBIFY® plus Standard Medical Treatment Compared to Placebo plus Standard Medical Treatment to Prevent Infections in Patients with Hypogammaglobulinemia and Recurrent or Severe Infections Associated with B-cell Chronic Lymphocytic Leukemia part II |
| 205. A PHASE 3, TWO-STAGE, RANDOMIZED, MULTICENTER, CONTROLLED, OPEN-LABEL STUDY COMPARING IBERDOMIDE MAINTENANCE TO LENALIDOMIDE MAINTENANCE THERAPY AFTER AUTOLOGOUS STEM CELL TRANSPLANTATION (ASCT) IN PARTICIPANTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM) (EXCALIBER MAINTENANCE) part I |
| 206. A PHASE 3, TWO-STAGE, RANDOMIZED, MULTICENTER, CONTROLLED, OPEN-LABEL STUDY COMPARING IBERDOMIDE MAINTENANCE TO LENALIDOMIDE MAINTENANCE THERAPY AFTER AUTOLOGOUS STEM CELL TRANSPLANTATION (ASCT) IN PARTICIPANTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM) (EXCALIBER MAINTENANCE) part I |
| 207. A randomized, double-blind, placebo-controlled multicenter study to evaluate the effect of inclisiran on preventing major adverse cardiovascular events in high-risk primary prevention patients (VICTORION-1 PREVENT) part I |
| 208. A randomized, double-blind, placebo-controlled multicenter study to evaluate the effect of inclisiran on preventing major adverse cardiovascular events in high-risk primary prevention patients (VICTORION-1 PREVENT) part II |
| 209. A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group, Dose-ranging Study to Evaluate the Efficacy and Safety of DC-806 in Participants with Moderate to Severe Plaque Psoriasis part I |
| 210. A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group, Dose-ranging Study to Evaluate the Efficacy and Safety of DC-806 in Participants with Moderate to Severe Plaque Psoriasis part II |
| 211. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study To Assess The Efficacy And Safety Of Rifaximin Soluble Solid Dispersion (SSD) Tablets For The Delay Of Encephalopathy Decompensation In Cirrhosis (RED-C) part I |
| 212. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study To Assess The Efficacy And Safety Of Rifaximin Soluble Solid Dispersion (SSD) Tablets For The Delay Of Encephalopathy Decompensation In Cirrhosis (RED-C) part II |
| 213. A Phase 2b Randomized, Double-Blind Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Efinopegdutide (MK-6024) in Adults with Precirrhotic Nonalcoholic Steatohepatitis part I |
| 214. A Phase 2b Randomized, Double-Blind Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Efinopegdutide (MK-6024) in Adults with Precirrhotic Nonalcoholic Steatohepatitis part II |
| 215. A randomized, open-label, multi-center phase III trial comparing tisagenlecleucel to standard of care in adult participants with relapsed or refractory follicular lymphoma part I |
| 216. A randomized, open-label, multi-center phase III trial comparing tisagenlecleucel to standard of care in adult participants with relapsed or refractory follicular lymphoma part II |
| 217. A Phase I, Open-label, Pharmacokinetic Study of TVB-2640 in Subjects with Mild, Moderate, or Severe Hepatic Impairment Compared to Subjects with Normal Hepatic Function part I |
| 218. A Phase I, Open-label, Pharmacokinetic Study of TVB-2640 in Subjects with Mild, Moderate, or Severe Hepatic Impairment Compared to Subjects with Normal Hepatic Function part II |
| 219. A PHASE II, MULTICENTER, RANDOMIZED, DOUBLE-BLIND STUDY OF RO7247669 COMBINED WITH NAB-PACLITAXEL COMPARED WITH PEMBROLIZUMAB COMBINED WITH NAB-PACLITAXEL IN PARTICIPANTS WITH PREVIOUSLY UNTREATED, PD-L1 - POSITIVE, LOCALLY-ADVANCED UNRESECTABLE OR METASTATIC TRIPLE-NEGATIVE BREAST CANCER part I |
| 220. A PHASE II, MULTICENTER, RANDOMIZED, DOUBLE-BLIND STUDY OF RO7247669 COMBINED WITH NAB-PACLITAXEL COMPARED WITH PEMBROLIZUMAB COMBINED WITH NAB-PACLITAXEL IN PARTICIPANTS WITH PREVIOUSLY UNTREATED, PD-L1 - POSITIVE, LOCALLY-ADVANCED UNRESECTABLE OR METASTATIC TRIPLE-NEGATIVE BREAST CANCER part II |
| 221. A Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy, Safety, and Pharmacokinetics of Baricitinib in Children from 6 Years to Less than 18 Years of Age with Alopecia Areata part I |
| 222. A Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy, Safety, and Pharmacokinetics of Baricitinib in Children from 6 Years to Less than 18 Years of Age with Alopecia Areata part II |
| 223. A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Patients in Patients With Hormone Receptor-Positive (HR+)/Human Epidermal Growth Factor Receptor 2-Negative (HER2-) (HER2-IHC0 or HER2-low [IHC 1+, IHC 2+/ISH-]) Inoperable, Locally Advanced, or Metastatic Breast Cancer and Have Received Endocrine Therapy part I |
| 224. A Randomized, Open-label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Patients in Patients With Hormone Receptor-Positive (HR+)/Human Epidermal Growth Factor Receptor 2-Negative (HER2-) (HER2-IHC0 or HER2-low [IHC 1+, IHC 2+/ISH-]) Inoperable, Locally Advanced, or Metastatic Breast Cancer and Have Received Endocrine Therapy part II |
| 225. A Multicenter, Open-label, Extension Study to Evaluate the Longterm Safety of Atogepant in Pediatric Subjects 6 to 17 Years of Age with Episodic Migraine part I |
| 226. A Multicenter, Open-label, Extension Study to Evaluate the Longterm Safety of Atogepant in Pediatric Subjects 6 to 17 Years of Age with Episodic Migraine part II |
| 227. A Randomized, Double-Blind, Phase 3 Study to Investigate the Efficacy and Safety of LY3437943 Once Weekly Compared to Placebo in Participants with Severe Obesity and Established Cardiovascular Disease (TRIUMPH-3) part I |
| 228. A Randomized, Double-Blind, Phase 3 Study to Investigate the Efficacy and Safety of LY3437943 Once Weekly Compared to Placebo in Participants with Severe Obesity and Established Cardiovascular Disease (TRIUMPH-3) part II |
| 229. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Pegozafermin in Subjects with Severe Hypertriglyceridemia (SHTG) part I |
| 230. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Pegozafermin in Subjects with Severe Hypertriglyceridemia (SHTG) part II |
| 231. A long-term extension study to evaluate the long-term safety, tolerability, and efficacy of subcutaneous amlitelimab in adult participants with moderate-to-severe asthma who completed treatment period of previous amlitelimab asthma clinical study part I |
| 232. A long-term extension study to evaluate the long-term safety, tolerability, and efficacy of subcutaneous amlitelimab in adult participants with moderate-to-severe asthma who completed treatment period of previous amlitelimab asthma clinical study part II |
| 233. A randomised, double-blind, parallel group, roll-over study evaluating long-term safety and efficacy of oral doses of BI 1291583 q.d. (Part A) followed by open label long-term safety assessment (Part B) in patients with bronchiectasis (ClairleafTM) part I |
| 234. A randomised, double-blind, parallel group, roll-over study evaluating long-term safety and efficacy of oral doses of BI 1291583 q.d. (Part A) followed by open label long-term safety assessment (Part B) in patients with bronchiectasis (ClairleafTM) part II |
| 235. A 2-Part, Open-Label, Phase 2, Multiple Dose Study to Evaluate the Pharmacodynamic Effects, Safety, and Tolerability of Patiromer in Children under 6 Years of Age with Hyperkalaemia (EMERALD 2) part I |
| 236. A 2-Part, Open-Label, Phase 2, Multiple Dose Study to Evaluate the Pharmacodynamic Effects, Safety, and Tolerability of Patiromer in Children under 6 Years of Age with Hyperkalaemia (EMERALD 2) part II |
| 237. A double-blind, randomized, placebo-controlled study to evaluate the efficacy, safety, and tolerability of intravenous ganaxolone added to standard of care in refractory status epilepticus part I |
| 238. A double-blind, randomized, placebo-controlled study to evaluate the efficacy, safety, and tolerability of intravenous ganaxolone added to standard of care in refractory status epilepticus part II |
| 239. A randomized, parallel-group, 24 week, double-blind, placebo-controlled, multicenter Phase 3 study to assess the efficacy and safety of secukinumab compared to placebo in adult patients with active rotator cuff tendinopathy part I |
| 240. A randomized, parallel-group, 24 week, double-blind, placebo-controlled, multicenter Phase 3 study to assess the efficacy and safety of secukinumab compared to placebo in adult patients with active rotator cuff tendinopathy part II |
| 241. A multicenter, randomized, prospective doubleblind, cross-over Phase 3 study to evaluate the efficacy and safety of 0.04 mmol Gd/kg body weight of gadoquatrane for MRI in adults with known or suspected pathology of any body region (except CNS), compared to 0.1 mmol Gd/kg approved macrocyclic gadolinium-based contrast agents (GBCAs) part I |
| 242. A multicenter, randomized, prospective doubleblind, cross-over Phase 3 study to evaluate the efficacy and safety of 0.04 mmol Gd/kg body weight of gadoquatrane for MRI in adults with known or suspected pathology of any body region (except CNS), compared to 0.1 mmol Gd/kg approved macrocyclic gadolinium-based contrast agents (GBCAs) part II |
| 243. An Open-Label, Phase 1 Study to Evaluate the Pharmacokinetics, Safety, Tolerability, and Pharmacodynamics in Response to a Single Subcutaneous Dose of Fazirsiran (TAK-999) in Subjects With or Without Hepatic Impairment part I |
| 244. An Open-Label, Phase 1 Study to Evaluate the Pharmacokinetics, Safety, Tolerability, and Pharmacodynamics in Response to a Single Subcutaneous Dose of Fazirsiran (TAK-999) in Subjects With or Without Hepatic Impairment part II |
Módosítási kérelmek
| Hónap | Kérelmek száma |
| Január | 83 |
| Február | 305 |
| Március | 225 |
| Április | 179 |
| Május | 232 |
| Június | 137 |
| Július | 144 |
| Augusztus | 190 |
| Szeptember | 142 |
| Október | 117 |
| November | (67) |
